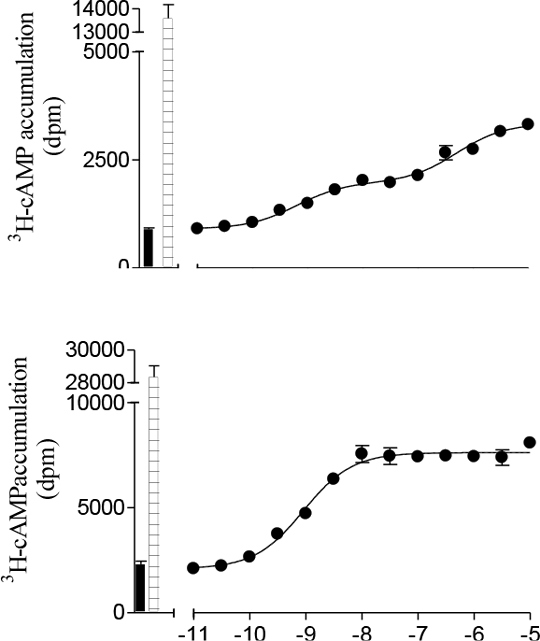
Determination of residues L195 and W199 as major contributors to the secondary low affinity conformation of the human β1-adrenoceptor. There are two active conformations of the β1-adrenoceptor (AR): a high affinity state where cimaterol is readily inhibited by antagonists and a low affinity state where CGP 12177 responses are relatively resistant to antagonism (Kaumann and Molenaar, 2008). We made single and triple point mutations in transmembrane 4 of the human β1-AR and examined the effects of these using CRE-SPAP production and 3H-cAMP accumulation in transiently transfected CHO-CRE-SPAP cells (Baker et al., 2008).
Log EC50, % max isoprenaline and log KD values at the WT and mutant receptors (CRE-SPAP assay) Cimaterol responses, and the affinity of antagonists were largely unaffected by the point mutations and the whole transmembrane (tm) 4 swap (see previous abstract for β1-tm4 details). CGP 12177 responses were more potent with the L195Q and W199Y mutations and the effects appeared additive. Antagonist affinity in the presence of CGP 12177 was greater with the V189T, L195Q and W199Y mutations, and again, the effects were additive. Pindolol, which has a biphasic response in the β1-WT due to stimulation via both the high affinity and low affintiy site (log EC501 -9.10 ± 0.04, log EC502 -5.93 ± 0.18, 57.1 ± 3.3% occurring at site 1, n=8), only had a single component response in the triple mutation (log EC50 -9.11 ± 0.03, n=4). In conclusion, the point mutations L195Q and W199Y have a major role and V189T a minor role in the secondary site of the human β1-adrenoceptor. Baker et al., (2008) Mol. Pharmacol. 74: 1246-1260 Kaumann and Molenaar (2008) Pharmacol. Ther. 118: 303-336. 3H-cAMP accumulation in response to pindolol.
 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||