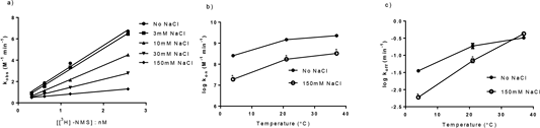
Effect Of Temperature And Ionic Strength On [3H]-NMS Binding To The Muscarinic M3 Receptor Studies determining the kinetics of clinically relevant compounds are often difficult to compare since differences in experimental conditions can significantly change receptor affinity and more importantly the kinetics of binding. This is evident in the field of M3 antagonists where the kinetic dissociation of tiotropium in non-physiological conditions is much slower than when performed under physiological conditions (Disse et al., 1993, Sykes et al., 2012). In this study we have determined the effect of salt and temperature on equilibrium affinity (K d) and kinetic parameters k on and k off of [3H]-NMS at the muscarinic M3 receptor. Affinity and binding kinetics were assessed using saturation and kinetic association methods (Sykes et al., 2009), at three temperatures; 4, 21 and 37ºC. A binding buffer consisting of 20mM HEPES pH 7.4, supplemented with 150mM NaCl where appropriate was employed and all reactions were terminated via rapid vacuum filtration through GF/B filter plates and radioactivity was quantified on a TopCount microplate scintillation counter. In association kinetic experiments at 37oC a gradual decrease (~10-fold) in in the magnitude of kon (M-1min-1) was observed as sodium chloride concentration was increased, there was however, no change in the value of koff (Figure 1a). Similar results have been observed in protein-protein interactions exposed to increasing ionic strengths (Schreiber & Fersht, 1993).

Figure 1. The effect of sodium chloride on the binding constants of [3H]-NMS kinetic. Data are Mean±SEM *P<0.05 2-way Anova Bonferroni t-test) . [3H]-NMS on and off-rates from the muscarinic M3 receptor markedly speed up when temperature is increased (Figure 1b and c), indicating an enthalpic contribution to the free energy of activation of the dissociation reaction. The inclusion of NaCl in the assay buffer reduced the association rate of [3H]-NMS at each temperature studied by a similar degree and also significantly reduced the rate of [3H]-NMS dissociation at the lower temperatures explored in this study. Understanding the effect of sodium chloride on kinetic rate constants is complex; investigations into other class A GPCRs such as the adenosine A2a receptor have shown that addition of sodium ions can result in an allosteric effect, slowing the off-rate of a radiolabelled antagonist (Gao & IJzerman, 2000). In this study [3H]-NMS equilibrium binding constants often showed little change as experimental conditions varied highlighting the importance of directly studying kinetic binding constants. Disse et al (1993) Life Sci 52 537-544. Gao & IJzerman (2000) Biochem Pharmacol 60 669-676 Schreiber & Fersht (1995) Biochemistry 32 5145-5150. Sykes et al (2009) Mol Pharm 76 543-551. Sykes et al (2012) JPET 343 520-528.
|