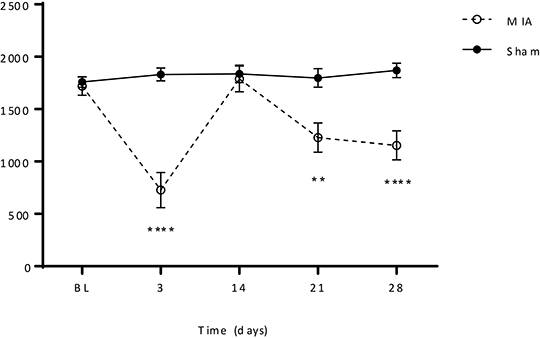
Characterisation of Burrowing Deficits in the Monosodium Iodoacetate (MIA) Rat Model of Osteoarthritis Pain The intra-articular injection of monosodium iodoacetate (MIA) into the rat knee joint induces histological changes in the cartilage and bone similar to those seen in clinical osteoarthritis (OA) with concomitant pain-related behaviours (1). The main techniques used to assess these pain-related behaviours are measures of evoked mechanical hypersensitivity, namely: deficits in weight bearing on the MIA-injected joint, mechanical hyperalgesia and mechanical allodynia (2). The aim of this study was to characterise deficits in burrowing behaviour as an objective non-evoked readout for pain in the MIA model and the sensitivity of these deficits to reversal by analgesics. Male Wistar Han rats (200-220g; Charles River, Germany) were used for all experiments. All animal experimental protocols were authorised by the Local Animal Care and Use Committee and carried out according to the local animal care guidelines. Steel burrows (32 cm x 10 cm) filled with 2.5 kg of quartz sand were used for all experiments and the amount of sand burrowed after 30 minutes of the rats being placed in the set-up was recorded. MIA (3 mg/knee in 50 μL 0.9% saline) was injected into both knee joints and burrowing was assessed 3, 14, 21 and 28 days later for the time-course study (Fig.1). Subsequent pharmacological experiments were carried out at the 3 day time point. Analgesics tested were: ibuprofen (3, 10 and 30 mg/kg, p.o.), celecoxib (3, 10 and 30 mg/kg, p.o.), the fatty acid amide hydrolase (FAAH) inhibitor PF-04457845 (10, 30 and 100 mg/kg, p.o.), morphine (0.3, 0.6, 1 and 3 mg/kg, s.c.) and an anti-nerve growth factor (NGF) monoclonal antibody (mAb) (9 mg/kg, s.c.). Drugs given p.o. were administered in 0.5% Natrosol and 0.1% Tween-80 (9:1 ratio) as vehicle with a pre-treatment time of 2 hours, morphine was administered in saline as vehicle and anti-NGF mAb in phosphate buffered saline as vehicle, with pre-treatment times of 1 and 24 hours, respectively. Bilateral injections of MIA resulted in deficits in burrowing performance 3, 21 and 28 days after injection compared to sham controls on the same day (P<0.0001, P<0.01, P<0.0001, respectively, n = 12). Deficits in burrowing performance at day 3 were reversible by ibuprofen (10 and 30 mg/kg, P<0.01 and P<0.05, respectively, n = 9-10), celecoxib (3, 10 and 30 mg/kg, P<0.01, P<0.05, P<0.01, respectively, n = 8-11) and anti-NGF mAb (9 mg/kg, P<0.01, n = 8), but not by morphine or PF-04457845. All P values for comparison to the MIA vehicle group.
Fig. 1. Time-course of burrowing deficits
In conclusion, burrowing is impaired by intra-articular injection of MIA and this impairment is reversible by the analgesics ibuprofen, celecoxib and an anti-NGF mAb. This validates the utility of burrowing as a preclinical assay of pain-related behaviour in the MIA model of OA. (1) Pomonis, JD et al. (2005). Pain 114: 339-346. (2) Fernihough, J et al. (2004). Pain 112: 83-93.
|