Print version
Search Pub Med
Mathematical Modelling of a Hollow Fibre Bioreactor for Systemic Toxicity Testing Current 2D in vitro test systems are poorly predictive of the toxicity of chemicals entering the systemic circulation. There is therefore an urgent need for models of systemic toxicity with improved predictivity across the pharmaceutical and chemical industry. We are currently developing a hollow fibre bioreactor (HFB) system for hepatotoxicity testing. Previously HFBs have shown promise for use as bioartificial livers and their use in hepatotoxicity testing is a natural extension to this work. To assist with the development of the HFB, the design has been mathematically modelled to inform its operating set up, interpret data from HFB outputs and aid in optimizing design to mimic certain hepatic physiological conditions. Additionally, the mathematical model has been used to identify the key HFB and compound parameters that will affect xenobiotic clearance. Methods Based on in vitro data, a cell-scale in silico cellular metabolism model was derived to describe the kinetics of cellular drug uptake, phase 1/2 metabolism and excretion of resulting metabolites. Most reactions took the form of Michaelis-Menten functions in systems of ordinary differential equations. The cell-scale model was then incorporated into a model for a functional liver sinusoid - represented as a cylindrical channel lined with individual hepatocytes which each cell having its own set of parameters to capture zonated variations of enzyme and transporter expressions. Sinusoid fluid flow was then incorporated as advection-diffusion rate equations. Whole body predictions were obtained by representing the liver as a collection of in silico sinusoids and incorporating this into a physiologically based pharmacokinetic (PBPK) framework.
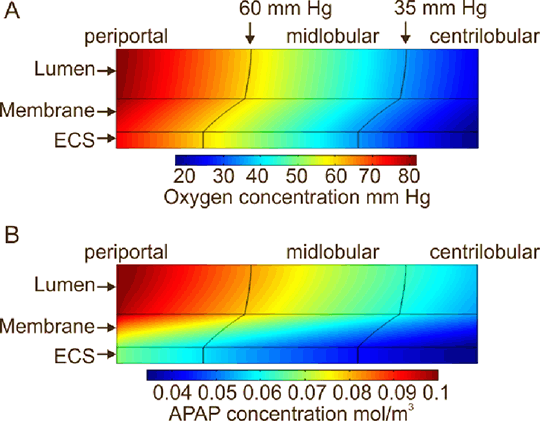
Figure 1: Computer simulation of concentration gradients of (A) oxygen and (B) APAP within a hollow fibre bioreactor seeded with primary rat hepatocytes with zonal regions indicated. Results The analysis of this model has produced novel analytical results that allow the operating set up to be calculated and predictions of compound clearance generated efficiently and in a highly accessible form. The mathematical model predicts the inlet oxygen concentration and volumetric flow rate that gives a physiological oxygen gradient in the HFB to mimic a liver sinusoid. It has also been used to predict the concentration gradients and clearance of a test drug and paradigm hepatotoxin, paracetamol (APAP). The effect of altering the HFB dimensions and fibre properties on paracetamol clearance under the condition of a physiological oxygen gradient is analysed. These theoretical predictions can be used to help design the most appropriate 3D in vitro experimental set up and data analysis to quantitatively compare the functionality of cell types that are cultured within the HFB to those in other systems.
|