Print version
Search Pub Med
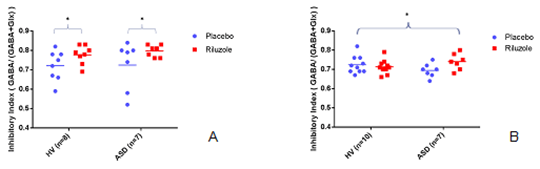
Glutamate and GABA in autism spectrum: a clinical in-vivo magnetic resonance spectroscopy assay There are currently no pharmacological treatments for the core symptoms of Autism Spectrum Disorders (ASDs). However, accumulating evidence indicates an imbalance between excitatory (E) glutamate and inhibitory (I) GABA- in favour of excitation- in ASD. Potentially, increasing inhibition by modulating glutamate and/or GABA may constitute a tractable treatment approach for ASD. We therefore conducted a Proof of Concept study to establish that there are differences in E/I flux in ASD compared to typically developing individuals. We used MEGAPRESS proton magnetic resonance spectroscopy ([1H]-MRS) to measure concentrations of Glx (glutamate + glutamine) and GABA in unmedicated adult men with and without ASD diagnosis. Individuals were scanned twice, one week apart. Fifty mg of riluzole or matched placebo was administered in a randomised, double blind, crossover design, 1 hour before spectra were acquired from the left basal ganglia and bilateral dorsomedial prefrontal cortex (DMPFC). An inhibitory index was calculated as GABA/(GABA + Glx) for both treatment conditions. Preliminary results show that, in the basal ganglia (Fig A), riluzole increased the inhibitory index in both groups (drug effect p=0.015, RM ANOVA). However, in the prefrontal cortex (Fig B), riluzole increased the inhibitory index in the ASD group only (drug*group effect p=0.038, RM ANOVA). Post-hoc testing suggested that increases in inhibitory indices in both groups were mainly driven by an increase in GABA, (and not a reduction in Glx). Data acquisition is ongoing. If confirmed, these preliminary findings bring us closer to Proof of Concept that individuals with ASD have differences in E/I responsivity. By validating the glutamate-GABA system as a tractable treatment target in ASD, we hope this work will both support trials of existing drugs (such as riluzole), and facilitate the development of new drugs that act on this system. Our MRS approach may also provide a safe, non-invasive tool to help fractionate the sample and predict who will be responsive to glutamate-GABA treatments in a ‘personalized’ medicine approach to ASD.
Figure 1. The ratio of GABA:Glx is lower in the basal ganglia in ASD compared to controls, which can be increased to control levels upon addition of riluzole
|
|||||||||||||