020P Queen Elizabeth II Conference Centre London
Pharmacology 2014 |
The Role Of EGFR-1 Signalling In Cardiac Microvascular Endothelial Cells
Bhaveshree Patel. Liverpool University, Liverpool, UK.
Endothelial cells (ECs) form the inner lining of blood and lymphatic vessels. Human cardiac microvascular endothelial cells (HCMECs) are found in myocardial capillaries. Their roles include paracrine signalling, oxygen and nutrient delivery to cardiomyocytes which enables cardiomyocytes to survive (1). Therefore, therapeutic drugs that potentially target them have the ability to cause cardiotoxicity. Currently, little is known about the effects of EGFR1 signalling in ECs as the majority of work is focused on EGF receptor expression in tumour cells. The aim of this study is to identify the roles of EGFR1 signalling in HCMECs and the effects of lapatinib, a dual tyrosine kinase inhibitor on these.
During the agonist stimulation HCMECs were stimulated with a variety of agonists at 50ng/ml for 10 minutes. Blots were probed for phospho-VEGFR2, phospho-EGFR1, phospho-Akt and phospho-ERK1/2. To quantify the fold increase in activation compared to basal following agonist stimulation, densitometry was carried out using ImageJ on the Akt and ERK1/2 bands of the agonist stimulation Western blot.
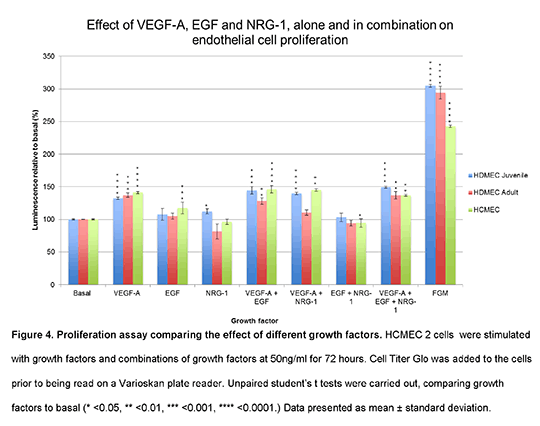
Real time quantitative PCR was then used to determine the mRNA expression of EGFR1 and its family members in HCMECs, dermal and coronary artery ECs. Cell line screening was then carried out to determine the extent of EGFR1 signalling in these EC lines. Cells were stimulated with VEGF or EGF at 50ng/ml for 10 minutes and blots were once again probed. Next, a proliferation assay (2) comparing the effects of different growth factors on proliferation was carried out. HCMECs were stimulated with growth factors and combinations at 50ng/ml for 72 hours. Cell Titer Glo was added to the cells prior to being read on a Varioskan plate reader. Unpaired student’s t tests were carried out, comparing each growth factor to basal to identify statistically significant data (* <0.05, ** <0.01, *** <0.001, **** <0.0001). Data presented as mean ± standard deviation (fig.4). Collagen tube assays were also carried out to identify tubular morphogenesis. HCMECs and dermal ECs were cultured on collagen and exposed to basal, VEGF, EGF, VEGF+EGF conditions at 50ng/ml for 24hours. For each cell line and condition, tube formation was compared to basal images. Finally, HCMECs were pre-treated with DMSO (vehicle control), 1µM lapatinib or 10µM lapatinib for 30 minutes and exposed to basal, VEGF and EGF conditions. Blots were probed. Results showed that HCMECs responded to EGF stimulation and activated downstream Akt and ERK1/2 cascades. Following this, HCMECs were shown to express EGFR1 mRNA. EGFR1s also showed functional roles in proliferation (fig.4) and tube formation in HCMECs. Finally, lapatinib inhibited EGFR1 signalling pathways in HCMECs. functional roles in proliferation (fig.4) and tube formation in HCMECs. Finally, lapatinib inhibited EGFR1 signalling pathways in HCMECs.
In summary, HCMECs express functional EGFR1s that couple to Akt and ERK1/2 signalling pathways which are involved in cellular survival and proliferation. Therefore, their inhibition by lapatinib may potentially explain the cardiotoxicity observed in patients.
(1) Aird WC (2007b). Circulation Research 100(2):174-190.
(2) Roberts OL (2010). Journal of Cell Sci 123(18): 3189-3200.
|