Print version
Search Pub Med
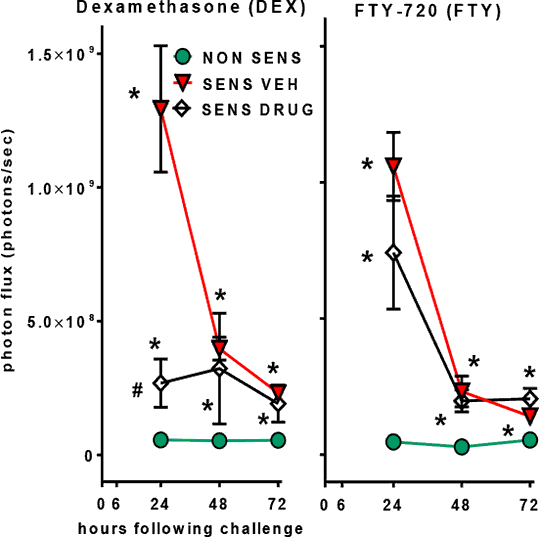
Non-Invasive Bioluminescence Imaging (BLI) of Inflammation in Murine Allergic Contact Dermatitis Experimental allergic contact dermatitis provides a simple means to study skin inflammation and immune responses as well as the inhibitory effects of drugs. The T-cell-mediated delayed type hypersensitivity (DTH) response manifests as an inflammatory reaction, due to activation of mononuclear phagocytes that reaches peak intensity 24 - 48 h after antigen challenge. In the present study, we aimed to develop non-invasive bioluminescence imaging as a standardized measure of inflammation in the oxazolone (OXA)-induced mouse DTH model. Systemic delivery of luminol enables detection of myeloperoxidase (MPO)-mediated bioluminescence (BL) largely mediated by tissue-infiltrating neutrophils (1,2). We compared this BL with inflammation assessed by ear thickness, by hematoxylin and eosin (H&E) stained skin sections and the Evans blue extravasation assay. In the OXA-induced dermatitis model (3), mice were sensitized by a single epicutaneous application of 100 μL of 3% OXA to the shaved abdomen (sensitization phase). Control mice received 100 μL of the vehicle (VEH), acetone:olive oil, 3:1, v/v. Seven days later, 20 μL of 1% OXA solution was applied to each side of one ear and 20 μL of vehicle to each side of the other ear (challenge phase). Firstly, we determined whether we could measure luminol bioluminescence in both CD-1 (Crl:CD1(ICR), white) and in C57Bl/6 (black) mice. Secondly, in separate treatment experiments, the glucocorticoids, dexamethasone and betamethasone (0.05 mg in 20 μL), were applied to each side of the ear (1h and 6h after the challenge with OXA). Thirdly, the sphingosine 1-phosphate (S1P) receptor modulator, FTY-720, was administered orally (p.o.) at 1 and 3 mg/kg (1h and 6h after the challenge with OXA). In all drug treatment studies, we used 9 week old male CD-1 mice (6-8 animals per group). Ear thickness was measured with a thickness gauge at baseline, 6h, 24h, 48h and 72h after the challenge. BL was assessed using the IVIS 200 bioluminescence imaging system (Perkin Elmer) and Living image software. Mice received luminol (200 mg/kg, i.p.) and were imaged at 24h, 48h and 72h after the challenge. Regions of interest (ROI) were manually selected at the application sites and radiance (total photon emissions) was determined. The Mann–Whitney U non-parametric test was used for statistical analyses. Luminol BL (photons/sec, mean ± SEM) of the OXA
Fig 1. Effects of dexamethasone (0.05 mg, locally) and FTY-720 (3 mg/kg p.o.) on BLI in OXA DTH. * p < 0.05 vs non-sens; # p < 0.05 vs sens VE (1) Gross S et al. (2010). Nature Medicine 15: 455–461. (2) Tseng J.-C. et al. (2012). Chemistry & Biology 19: 1199–1209. (3) Mimura T et al. (2012). Life Sciences 90: 862–866.
|