The Molecular Basis for the Effects of RAMP1 on the Calcitonin Receptor Receptor activity modifying proteins (RAMPs) are a family of proteins that interact with G-protein coupled receptors (GPCRs). When the calcitonin receptor (CTR) interacts with RAMP1, there is a marked increase in affinity for the peptide hormone amylin (1). The molecular basis for this is unclear. In the current study, residues have been identified in the extracellular domain (ECD) of the human CTR that become important for amylin binding after the interaction with RAMP1 using alanine mutagenesis. Molecular modelling has been used to suggest a mechanism for the action of RAMP1. Mutagenesis and receptor expression in Cos 7 cells was as described previously (2). Concentration-response curves were constructed to rat amylin and cAMP was measured with Alphascreen on wild-type and mutated receptors. Data was analysed by Prism 6 using a three-parameter fit to obtain pEC50 values. Values were compared to controls by Student’s t- test. Homology models of the ECD of the CTR alone and docked to the ECD of RAMP1 were constructed using Modeller, using the structure of the CLR/RAMP1 ECD complexes (2N7R) as a template (2). After minimisation, they were subjected to 1 microsecond molecular dynamics simulations. Principal component analysis of the resulting trajectories was used to identify the main eigenvectors and the root mean square fluctuation (RMSF) calculated.
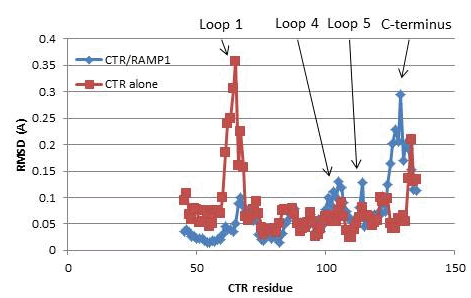
Table 1. Effects of alanine mutants on pEC50 for amylin on cAMP production on the CTR/RAMP1 complex, n=4-5. Fig 1. Changes in RMSF
W79, D101, F102, R126, W128 and Y131 all made substantial contributions to amylin potency (Table 1). These are found on loop 4 and the C-terminus of the ECD of CTR. Whilst over 10A from the RAMP1 interface, these parts of CTR become much more mobile on association with RAMP1, as shown by an increase in RMSF (Fig 1). RAMP1 may act allosterically to increase the dynamics of CTR. This work was supported by the Wellcome Trust (091496). 1) Christopoulos G et al. (1999). Mol Pharmacol 56:235-43 2) Watkins HA et al, (2014). Br J Pharmacol 171: 772-88 |