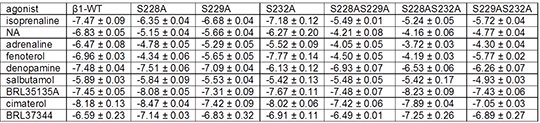
Impact of serine to alanine mutations in transmembrane five of the human β 1-adrenoceptor on agonist-stimulated cAMP responses. RGW Proudman, JG Baker. University of Nottingham, Nottingham, UK. Mutagenesis studies have determined that there are three serines at positions 203, 204 and 207 in transmembrane five of the β2-adrenoceptor that are important for binding the hydroxyl (OH) groups of catecholamines. Whilst the meta-OH of catecholamines binds to S203 and S204, the para-OH binds to S207 -2. The equivalent serines in the β1-adrenoceptor are at positions 228, 229 and 232 and affect isoprenaline affinity [4]. Here, cells stably expressing the human β1-wildtype (WT) receptor or cells stably expressing β1 receptors with serine to alanine mutations S228A, S229A and S232A, either alone or in combination, were studied. Agonist responses to catecholamines (isoprenaline, noradrenaline (NA), adrenaline), agonists with similarly positioned hydroxyl groups (fenoterol, salbutamol, denopamine) and those without hydroxyl groups (cimaterol, BRL35135A, BRL37344) were examined using 3H-cAMP accumulation as previously described [4].
Table. Log EC50 values for the responses to agonists obtained from 3H-cAMP accumulation in cell lines stably expressing the β 1-WT receptor or a β 1-receptor with one or two serines (S) mutated to alanine (A). Values are mean ± s.e.m. for 4 separate experiments in each case. Catecholamine responses were all less potent in the β1-S228A, β1-S229A, β1-S232A mutants than the β1-WT cells. S228A affected the response to fenoterol (which has meta-OH groups) but not denopamine or salbutamol (both have para-OH groups). S232A affected the response to denopamine (para-OH) but interestingly not that of salbutamol (also para-OH). Responses to ligands without the hydroxyl groups were largely maintained. For receptors with two serine mutations, responses to the catecholamines were further reduced although responses were still seen with loss of two of the three serines. Fenoterol responses were most affected by the double mutations containing S228A and denopamine by those containing with S232A. In conclusion, the serines at positions 228, 229 and 232 affect the potency of agonist response to catecholamines and other agonists containing hydroxyl groups [1] Liapakis G, et al., (2000). J Biol Chem. 275: 37779-37788. [2] Sato T, et al., (1999). Br J Pharmacol. 128: 272-274 [3] Strader CD, et al., (1989). J Biol Chem. 264: 13572-13578. [4] Baker JG, et al (2008). Mol. Pharmacol. 74: 1246-1260.
|