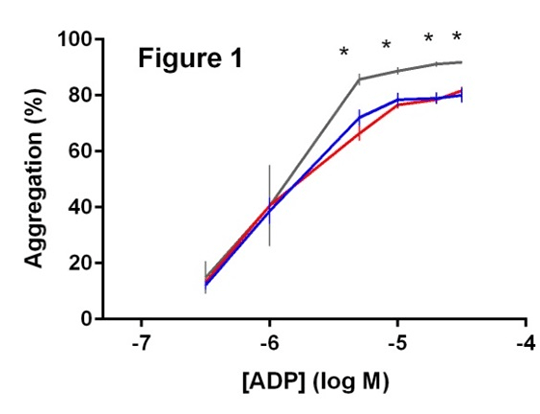
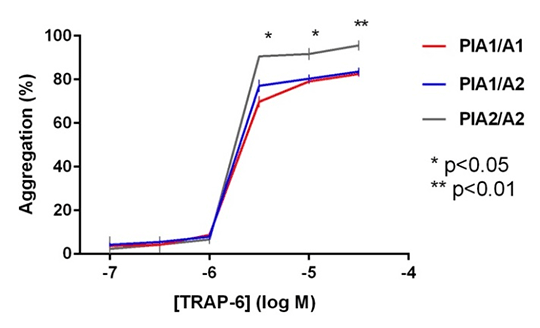
The PlA2 Allele of Glycoprotein IIIa and the Impact of Carriage on Platelet Function Introduction: Carriage of the mutant PlA2 allele of glycoprotein IIIa (GPIIIa) confers an increased risk of myocardial infarction and ischaemic stroke -1. The allele encodes a single amino acid substitution adjacent to the ligand binding site of GPIIIa, but it is currently unclear as to how this substitution affects platelet function. Here we investigate the proteomics of PlA2 expression and how carriage of PlA2 impacts on platelet function in patients with cardiovascular disease. Methods: Ninety patients with stable coronary artery disease prescribed daily 75mg aspirin underwent analysis of platelet proteomics and function. Carriage of the PlA2 allele was assessed through the development of a liquid chromatography, mass spectrometry (LC-MS/MS) assay that enabled absolute quantification of GPIIIa expression. The presence of the wild-type (PlA1) and mutant (PlA2) alleles were inferred through the presence of prototypic peptides detected above the lower limits of the assay, with appropriate time-aligned transitions of peptide fragmentation. Platelet function was assessed in platelet-rich plasma by Optimul in response to arachidonic acid (AA; 0.03-1.6mM), adenosine diphosphate (ADP; 0.3-30µM), collagen (0.1-30μg/mL), adrenaline (0.001-100μM), ristocetin (0.2-10mg/mL), thrombin receptor activating peptide 6 (TRAP-6; 0.1-30μM) and the thromboxane receptor agonist U46619 (0.1-30μM) [3]. Statistical analyses were performed using Mann-Whitney test, with significance at p<0.05. Results: Sixty nine patients were homozygous for wild-type, two were homozygous for mutant and 19 expressed both wild-type and mutant peptides. Demographic, haematological and biochemical characteristics between the groups were not different. The overall prevalence for carriage of the PlA2 allele was 23%. Mendelian regulation of expression was observed, with 1:1 expression of wild-type and mutant peptides in platelets from all heterozygous patients. Analysis of platelet function revealed that for all agonists except AA, aggregation in response to higher agonist concentrations was significantly increased for PlA2/A2 patients compared to PlA1/A1 (all p<0.05; Figure 1). No significant difference in aggregation was observed for PlA1/A2 versus PlA1/1. Conclusions: The expression of the PlA1/A2 alleles of GPIIIa in platelets conforms to Mendelian patterns. Patients homozygous for the mutant allele have increased levels of platelet reactivity to multiple agonists and this may underlie their increased risk of cardiovascular events.
(1) Floyd et al. (2012) PLoS One 9: e100239. (2) Floyd et al. (2012) PLoS One 9: e101518. (3) Chan MV, Warner TD. (2012) Platelets 23: 404-8.
|