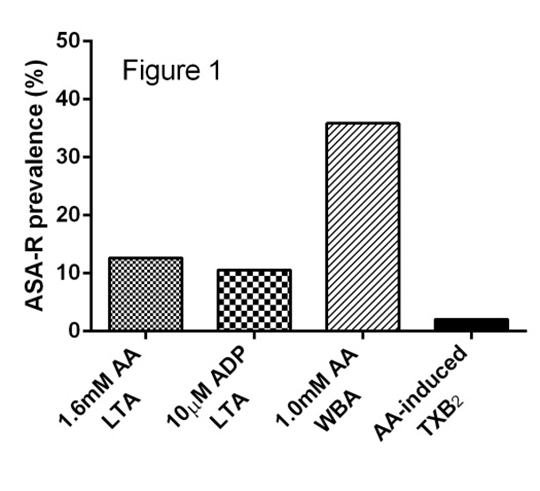
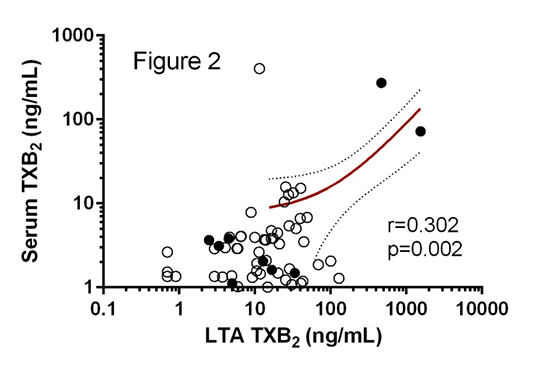
The Role of Biochemical Tests in the Assessment of Aspirin Resistance Introduction: Aspirin is integral to the secondary prevention of cardiovascular disease, but a proportion of patients fail to appropriately respond to therapy in a heterogeneous phenomenon known as aspirin resistance (ASA-R) [1]. ASA-R status is defined based on platelet function tests and/or the presence of thromboxane A2 metabolites in either serum or urine. However, with the exception of platelet-rich plasma (PRP) derived thromboxane B2 (TXB2), all assays are effectively surrogates for platelet cyclooxygenase-1 (COX-1) inhibition. Here we investigate how biochemical tests correlate with functional assessments of ASA-R status. Methods: Whole blood was drawn into citrate (final concentration 0.32%) from patients with stable coronary artery disease prescribed aspirin 75mg daily (n=95). Light transmission aggregometry (LTA) was performed in platelet-rich plasma in response to 1.6mM arachidonic acid (AA; the ‘gold standard’ test; ASA-R ≥20% aggregation) and 10μM adenosine diphosphate (ADP; ASA-R ≥70% aggregation). Whole blood aggregometry was performed in response to 1.0mM AA (ASA-R ≥3Ω impedance). TXB2 levels were measured in supernatant following AA-induced LTA (ASA-R ≥216ng/mL), and in serum from whole blood following unstimulated coagulation in borosilicate (37oC for 1h). Inter-assay agreement was compared to 1.6mM AA-induced LTA using κ statistic. Spearman’s correlation and ROC analysis were used to assess the paired TXB2 samples. Results: All patients reported medication concordance with aspirin consumption <6h pre-phlebotomy. The prevalence of ASA-R varied based on assay as shown in Figure 1. TXB2 levels normalised in the two biochemically ASA-R patients following incubation with 100μM ASA, suggesting a pharmacokinetic mechanism for ASA-R [2]. Inter-assay agreement of ASA-R status was poor for ADP-induced LTA (κ=0.134) and WBA (κ=0.134), but fair for AA-induced TXB2 (κ=0.259). TXB2 levels from AA-induced LTA and unstimulated clotted blood demonstrated low correlation (Figure 2; closed circles ASA-R by AA-induced LTA), although ROC analysis for unstimulated TXB2 produced an AUC=0.980 (p=0.020). Conclusion: Biochemical ASA-R is rare and has generally poor agreement with functional definitions of ASA-R. Despite TXB2 generation in unstimulated whole blood having a low correlation with AA-induced TXB2, it appears to have utility in the diagnosis of biochemical ASA-R.
(1) Floyd CN et al. (2014) Pharmacol Ther 141: 69-78. (2) Weber A et al. (2002) Platelets 13: 37-40
|