Print version
Search Pub Med
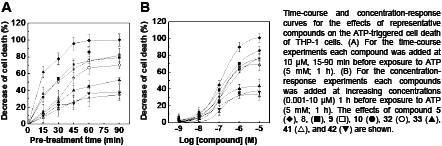
Design, Synthesis and Pharmacological Evaluation of Anti-Pyroptotic Compounds Acting on NLRP3 Inflammasome Signaling Pathway: a New Start for Anti-Inflammatory Drugs? Pyroptosis is a caspase-1-dependent pro-inflammatory form of programmed cell death implicated in the pathogenesis of autoinflammatory diseases known as cryopyrin-associated periodic syndromes (CAPS), as well as in disorders characterised by excessive cell death and chronic inflammation, such as rheumatic and neurodegenerative diseases (1, 2). Activation of NLRP3 inflammasome is a key event in the regulation of the pyroptotic cascade. The aim of this study was the design of new compounds able to interfere with NLRP3-promoted pyroptotic cascade. A library of 36 compounds was synthesised using an electrophilic fragment-based strategy. Pyroptosis was studied in phorbol myristate acetate-differentiated and LPS-primed THP-1 cells (a model of activated human macrophages) exposed to ATP (5 mM; 1 h) or nigericin (10 μM, 1.5 h). Pyroptotic cell death was evidenced through microscopic examination of cellular morphology and evaluated by measuring the increase in lactate dehydrogenase activity in the collected supernatants. Pyroptosis of THP-1 cells was significantly prevented (p<0.05 vs. vehicle alone, ANOVA and Bonferroni’s post-test) by most of the synthesised compounds with an efficacy ranging from 12.1±1.2% to 97.9±5.7%. Cytotoxicity of new compounds was evaluated by measuring viability of human renal epithelial cells (HK-2) by MTT assay. The anti-pyroptotic effect proved to be both time- and dose-dependent with best performing compounds exhibiting EC50 values in the submicromolar range. The ability of model compounds to react, in vitro, with biologically relevant thiol groups was then proved using 1H-NMR spectroscopy and gluthatione as the probe. Preliminary insights into the mechanism of action of selected derivatives showed that they prevent pyroptosis by interfering with the NLRP3 inflammasome function. In conclusion, our results not only identify a molecular scaffold suitable for developing druggable compounds, but also indicate the role of reactive cysteine residues in the NLRP3 inflammasome regulatory mechanisms.
(1) Dinarello CA et al. (2012). Nat Rev Drug Discov 11: 633-652. (2) So A et al. (2013). Nat Rev Rheumatol 9: 391-399.
|