Print version
Search Pub Med
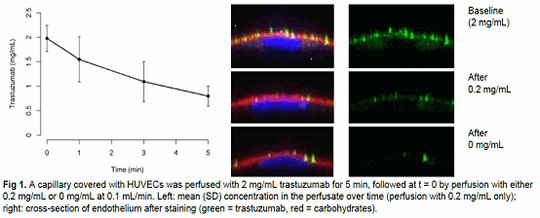
Biotherapeutics: How Much Do We Actually Administer And Where Does It Go? In various disease areas, biotherapeutics (BTs) are of increasing importance. Commonly, therapeutic efficacy is linked to plasma drug concentration and thus to pharmacokinetic (PK) properties. However, when administered intravenously, some BTs, such as trastuzumab, bevacizumab, and C1-inhibitor, are observed to reach maximal plasma concentration at a time beyond the infusion duration. To elucidate this phenomenon, a series of in vitro experiments mimicking intravenous drug administration was conducted, based on the hypothesis that the vasculature dynamically binds and releases BTs depending on local concentration. METHODS To study whether endothelial cells (ECs) are capable of binding therapeutic proteins, human umbilical vein ECs (HUVECs) were exposed to different concentrations of multiple BTs, including monoclonal antibodies. ECs were first cultured for 7 days under flow, to optimally mimic the in vivo situation. Furthermore, incubations with BTs were performed under static conditions to determine the protein's adhesion to the endothelium, and under flow to dynamically study BT adhesion and release. After the incubations, HUVECs were stained with a fluorescent secondary antibody for quantification. The concentration of the BT was determined in the perfusate for the experiments focusing on protein release.
RESULTS Initial results indicate that BTs can adhere to ECs in a concentration-dependent manner, explaining the delayed rise in BT plasma concentration observed after intravenous infusion in vivo (Fig 1). Since the adhered BTs could be easily removed by washing, this binding seems to be non-specific, and most likely electrostatic. Endocytosis or migration across an intact endothelial barrier was not observed. DISCUSSION We demonstrate that the endothelium could serve as a vast reservoir that non-specifically and temporarily captures BTs for delayed release into the circulation after cessation of the infusion. This binding could hamper the interpretation and comparability of results of clinical trials in terms of efficacy, PK and untoward effects. It may also result in under- or overdosing of individual patients, depending on the state of the endothelium, the available surface area, the administration conditions, and the used infusion materials. Additionally, (local) adhesion to the endothelium could modulate its function. As this is an ongoing series of experiments, the latest results will be presented at the conference.
|