Print version
Search Pub Med
The Functional Expression of Uterine FP Receptors in Wild Type and Fatty Prostamide/ Prostaglandin F Synthase Knockout Mice Prostamide/ prostaglandin F synthase (PM/PGFS) is an enzyme that catalyses the conversion of prostamide H2 to prostamide F2α as well as prostaglandin (PG)H2 to PGF2α (1). PGF2α and prostamide F2α are known to be anti-adipogenic (2) but have contrasting effects on the uterus (3). To further characterise its physiological significance, for the first time PM//PGFS-deficient mice were used. Isolated tissues were obtained from female C57 wild type (n=5) and knockout mice (n=7) with ad libitum access to food. Uterine strips were immersed in Krebs’ solution as previously described (3). Responses to vehicle, PGF2α (10-9M to 10-5M) and bimatoprost (10-6M), a prostamide mimetic (4), were measured against a reference hypotonic shock contraction. Each agonist was solubilised in ethanol and diluted with saline (0.9% w/v). The uterus, omentum and subcutaneous fat were also snap frozen for real-time PCR analysis of FP transcripts. Data are expressed as means ± S.E.M and analysed using a Student’s t-test or ANOVA with Bonferroni’s post-hoc adjustment. Table 1: Age and weight of mice at time of sacrifice; *p<0.05 vs wild type
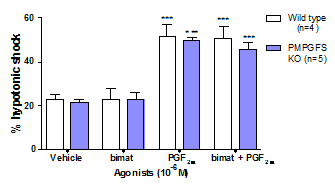
Figure 1: Effect of vehicle, bimatoprost (10-6M) and PGF2α (10-6M) on uterine activity; ***p<0.001 vs vehicle
PM/PGFS knockout mice were heavier than wild type mice of the same age (p<0.05; Table 1). Although this was manifest as fat deposition, each strain showed comparable uterine activity and responsiveness to agonists (Figure 1). PGF2α enhanced contractions 2-fold over time-matched vehicles (p<0.001) whilst bimatoprost (10-6M) had no effect. Similarly gene deletion did not influence transcription of FP receptors in mouse uterine or adipose tissues. The knockout mouse model results are consistent with in vitro studies on prostamide F2 α mediated inhibition of pre-adipocyte differentiation, suggesting that PM/PGFS could be an important inhibitor of adipogenesis. Despite the oestrogenic capacity of fat deposits (5), uterine contractions and functional expression of FP transcripts remained constant. Prostamide F2α signalling therefore appears to modulate adipose metabolism without affecting the uterus. (1) Moriuchi H et al. (2008). J Biol Chem 283: 792-801. (2) Silvestri C et al. (2013). J Biol Chem 288: 23307-23321. (3) Chen J et al. (2005). Br J Pharmacol 144: 493-501. (4) Woodward DF et al. (2008). Br J Pharmacol 153: 410-419. (5) MacKenzie SM et al. (2008) Clin Endocrinol 69: 848-854.
|