Print version
Search Pub Med
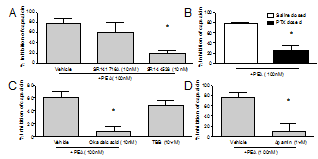
Determining The Signalling Pathway Mediating Endocannabinoid Inhibition Of Airway Sensory Nerve Depolarisation And The Cough Reflex Cough is an essential defensive reflex event, however excessive/chronic cough can be a problematic symptom of respiratory diseases, for which no effective and safe medications are available1. Fatty acid amide hydrolase (FAAH) is an enzyme that catalyses the hydrolysis of amidated signalling lipids. FAAH inhibition elevates endocannabinoid levels (including palmitoylethanolamide, anandamide, oleoylethanolamide and linoleoylethanolamide: PEA, AEA, OEA, LEA), causing inhibition of citric-acid evoked cough in guinea pigs, and PEA inhibits sensory nerve depolarisation2. Here we investigate the signalling pathway involved in endocannabinoid inhibition of sensory nerve/neuron activity. Isolated vagal nerves or neurons were dissected from male Dunkin-Hartley guinea pigs (300-500g, Harlan) or human lung (unsuitable for transplant)3,4. PEA (1-1000nM) inhibited capsaicin-induced depolarisation of guinea pig vagus nerve (max 84.2±6.7% n=4). PEA also inhibited capsaicin-induced calcium flux in guinea pig airway-terminating jugular neurons (62.7±4.9% N=3). A CB2 (SR144528) but not CB1 (SR141716A;) antagonist (10nM) reversed PEA inhibition in guinea pig and human vagus depolarisation (fig 1a). Similarly the endocannabinoids AEA, OEA and LEA (100nM) also inhibited depolarisation (~70%) of guinea pig vagus in a CB2 dependent manner. Furthermore, PEA no longer inhibited depolarisation in guinea pigs dosed 3 days prior (25μg i.p.) with pertussis toxin (fig 1b), indicating a role for the G-protein αi subunit. Apamin (1μM: SKCa blocker) reversed PEA inhibition of guinea pig and human vagus depolarisation (fig 1c). Furthermore, PEA inhibited depolarisation to the TRPA1 agonist acrolein (300μM;79.7±6.9%, n=3 p<0.05 paired t test) and the TRPV4 agonist GSK1016790a (300nM;64.2±10.7%, n=3 p<0.05 paired t test) indicating a general suppression of depolarisation concurrent with enhanced SKCa activity. PP2A and CK2 increase and decrease the calcium sensitivity of SKCa 5. Okadaic acid (10nM; PP2A inhibitor) blocked PEA inhibition of guinea pig vagus depolarisation, and additionally, TBB (10μM:CK2 inhibitor) itself inhibited depolarisation (fig 1d). Together this data suggests the endocannabinoid PEA inhibits sensory nerve activation in guinea pig and human vagus nerve via a CB2/Gi/PP2A/SKCa pathway. Furthermore, this data implies that inhibiting FAAH/elevating endocannabinoids may be beneficial in conditions driven by excessive peripheral sensory nerve activation including chronic cough.
NB – drug vehicle 0.1% DMSO unless stated, sub-maximal conc of PEA (100nM) or capsaicin (1μM) used unless stated. Groups compared to vehicle controls using Mann-Whitney U or Kruskall-Wallis w/Dunns post-hoc, p<0.05 considered significant. 1. Nasra J and Belvisi MG (2009). Pharmacol Ther 124: 354–375 2. Adcock, J. J. et al. (2011).Proc Br Pharmacol Soc. 9: A025p 3. Belvisi, M. G. et al. (2009). Br J Pharmacol 155: 547–557 4. Dubuis ED et al. (2013). Curr. Protoc. Pharmacol. 62: Unit 12.15 5. Adelman JP et al. (2012). Annu. Rev. Physiol. 74: 245–69
|