Print version
Search Pub Med
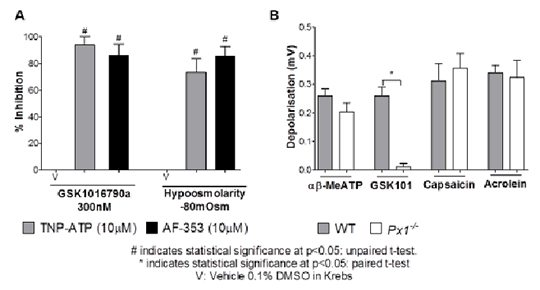
TRPV4 And Activation Of Airway Sensory Nerves: The Role Of ATP The TRPV4 channel is a member of the Transient Receptor Potential family of ion channels. It is expressed in the airway and is involved in responses to stimuli such as changes in pH and osmolarity. We have previously shown that activation of TRPV4 by the small molecule activator GSK10167090a causes depolarisation of the vagus nerve, single airway fibre firing and reflex cough in the conscious guinea pig through activating nodose derived Aδ airway fibres(1). Both calcium signal and fibre firing demonstrated a short delay prior to activation following application of GSK101, suggesting the release of a secondary mediator. Activation of TRPV4 on bladder afferents has previously been linked to ATP release and the purine receptor P2X3 (2). Furthermore, a recent clinical study has shown that a P2X3 antagonist AF-219 caused a significant decrease in daytime objective cough frequency in patients with treatment resistant chronic cough (3), implicating the involvement of this pathway. Therefore the aim of this study was to investigate the mechanism of TRPV4 mediated activation of airway sensory nerves, and the role of ATP and P2X3. Using RTPCR of whole male guinea pig ganglia, the purine receptor P2X3 was expressed predominantly on the nodose ganglia. Calcium (FURA-2) imaging of airway specific neurons isolated from male Dunkin Hartley guinea pigs (4), indicated that αβ-MethyleneATP (αβ-MeATP; 10-100μM), a more stable agonist at P2X1 and P2X3 containing receptors, induced calcium flux in the nodose ganglia (n=3), alongside causing depolarisation of both guinea pig (n=4) and donor human tissue (n=3) in a well characterised in vitro vagal nerve preparation (5). Depolarisation of human and guinea pig sensory nerves in vitro by the TRPV4 activators GSK1016790a (300nM) and hypoosmolarity (-80mOsm) was inhibited following the use of two selective P2X3 antagonists AF-353 (10µM) and TNP-ATP (10µM) (Fig 1A). TRPV1 and TRPA1 responses remained unchanged. A mechanism for ATP release was indicated as GSK1016790a induced depolarisation was abolished in Pannexin 1 knockout mice (Px1-/- ), however depolarisation induced by αβ-MeATP and TRPV1 and TRPA1 agonists remained unchanged, indicating that TRPV4 initiates ATP release through the Pannexin 1 pore in mice (n=4; Fig 1B). In vivo, firing of single airway-innervating fibres was measured as previously described (6), and Aδ fibre firing induced by GSK1016790a (10ng/ml) was significantly inhibited following AF-353 (10mg/kg) administration (frequency of firing was reduced from 16.67±4.4imp/s to 4±1.5imp/s, n=3 p<0.05 paired t-test). Finally, cough induced by GSK1016790a (30µg/ml) was also inhibited by AF-353 (10mg/kg) to a similar extent as by the TRPV4 antagonist GSK2193874 in the guinea pig (n=8).
(1) Belvisi MG et al (2013) AJRCCM A5265. (2) Aizawa N et al.(2012) Neurourol Urod 1:148-55. (3) Abdulqawi et al (2013) ERJ 42. 57 1965. (4) Dubuis E. et al.(2013)Curr Prot Pharm 62:12.15. (5) Birrell MA et al.(2009)AJRCCM 180:1042–1047 (6) Adcock J.J et al.(2003) BJP 138:407–416
|