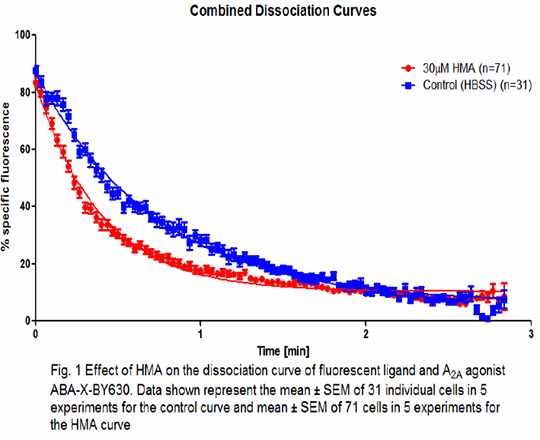
Use of fluorescent ligands to study ligand-binding kinetics and allosterism at the human adenosine A2A receptor Allosteric modulation of G protein-coupled receptors is a promising and relatively new field of drug discovery. The characteristics which allosteric modulators possess, such as high specificity to a single receptor and the lack of inherent activity without an orthosteric ligand, means they have the potential to be developed into safer and more efficacious drugs (1). Allosteric modulators of the adenosine A2A receptor, such as 5-(N,N-Hexamethylene)amiloride (HMA) (2), have been previous described. The aim of this study was to determine the properties of HMA at the adenosine A2A receptor in living cells using a fluorescent agonist. CHO CRE-SPAP cells expressing the human adenosine A2A receptor were used to characterise the fluorescent adenosine receptor agonist ABA-X-BY630 and the effect of HMA on agonist (NECA) stimulated responses in the CRE-SPAP gene transcription assay as described previously by Vernall, et al. (3). All confocal microscopy was performed on CHO cells transiently expressing A2A-YFP and using a Zeiss 710 confocal microscope. Perfusion confocal microscopy as described by May et al (4) was utilised to study the ligand binding kinetics of ABA-X-BY630 on the A2A receptor under the influence of HMA. It was confirmed that the fluorescent ligand ABA-X-BY630 is an agonist at the adenosine A2A receptor (pEC50 = 7.50 ± 0.17, n=4). Confocal imaging showed that ABA-X-BY630 had distinct membrane localisation and binding was blocked in the presence of an unlabelled antagonist (pre-incubated with 10 μM XAC for 30 minutes). Kinetic analysis of the binding of ABA-X-BY630 yielded values for kon = 5.0x106±3.7x105 M-1.min-1 and koff =1.4±0.04 min-1 (n=4; calculated pKD = 6.55). The presence of 30 µM HMA caused a significant increase in the dissociation rate of the fluorescent ligand compared to the control curve (koff: 2.5±0.04 min-1; P value < 0.0001) (Figure 1). In the functional assay, 30 µM HMA decreased the Emax of the orthosteric agonist NECA from 93±6 to 64±6% but had no effect on the potency of the agonist (pEC50 = 8.0±0.2 (NECA) vs. 8.0±0.3 (NECA+30 μM HMA), n=3). In summary, HMA increases the dissociation rate of an orthosteric fluorescent agonist and causes a reduction in the efficacy of the agonist NECA in a functional assay and taken together this suggests that HMA negatively modulates the adenosine A2A receptor in living cells.
(1) Wootten D et al., (2013) Nat Rev Drug Discov. 12, 630-44 (2) Gao, ZG and IJzerman AP. (2000) Biochem. Pharmacol. 60, 669–76 (3) Vernall, AJ et al., (2012) J. Med. Chem. 55, 1771–82 (4) May, LT et al., (2010) Mol. Pharmacol. 78, 511–23 This work has received support from the EU/EFPIA Innovative Medicines Initiative Joint Undertaking, K4DD grant n° 115366. |