Print version
Search Pub Med
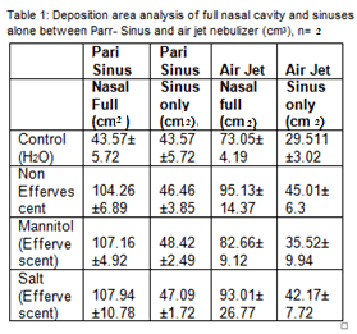
Study of deposition patterns of effervescent liposomes via Pari-Sinus and Air-Jet nebulizers using twin Impinger and Nasal cast model Introduction and Purpose: Although sinusitis conditions are quite common, there are severe limitations in existing drug delivery mechanisms to the nose and paranasal sinuses (1). This is mostly due to the location of hidden pockets and the fact that para-sinuses are non-ventilated (1). A number of devices have been examined for the effective delivery of aerosol. This study investigates effectiveness of incorporating the effervescent ingredient in liposomes along with the drug, to increase drug deposition in para-sinuses (2). Pari-Sinus and Air Jet nebulizers have been compared on the basis of their ability to deliver aerosols to the nasal cavity and sinuses. Method: Effervescent proliposome formulations were manufactured and analysed with cholesterol, and by using mannitol or effervescent salts (Sodium Bicarbonate, Sodium Benzoate and Citric Acid). Effervescent proliposome formulations with Beclometasone Dipropionate (BDP) were hydrated in water and nebulised using Pari-Sinus and Air Jet nebulizers. A unique system was developed using an twin impinger and a transparent nasal cast model coated with Sar-Gel® (water indicating paste). Nebulization sprays were then photographed and images quantified using Adobe® Photoshop, pixels were counted and then converted it in to area cubic centimetre for both full nasal cavity and sinuses deposition area. Results: The deposition area for the ful
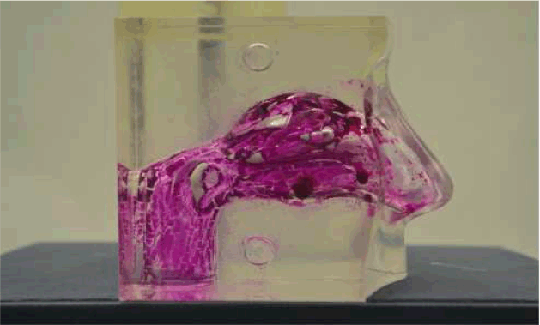
Figure 1: Full nasal cavity deposition of liposomal formulation by Pari-Sinues nebulizer Conclusion: Liposomal formulation depositions Reference: (1) O.D Korale, et.al.,2013. Proceeding 2013 AAPS Annu. Meet. Expo. November. 10-14 2013 San Antonio TX Vol. Poster R6163. (2) O. D. Korale, et.al., 2014. Presented at the Academy of Pharmaceutical Sciences (APS UKPharmSci 2014), University of Hertfordshire, Hatfield, UK.
|