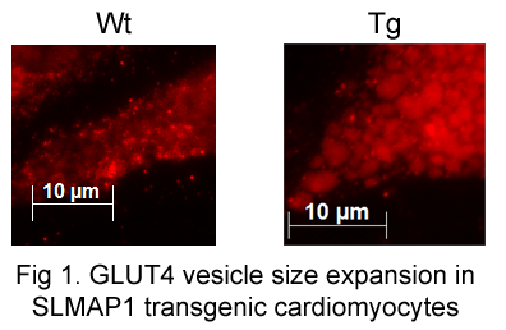
Sarcolemmal Membrane Associated Protein (SLMAP): Novel Regulator of Glucose Metabolism and Target In Cardiac Disorders The glucose transporter 4 (GLUT4) is critical for glucose transport into cells and its regulation and trafficking is of much interest in diabetes (1).Studies have shown the increased blood glucose levels associated with diabetes can be reversed by increasing the expression of this transporter thus preventing insulin resistance and diabetes. Sarcolemmal membrane associated protein isoform 1 (SLMAP1) is highly expressed in heart tissue and has been shown to play a role in excitation-contraction coupling and regulation of GLUT4 levels (2, 3, 4, 5). GLUT4 and SLMAP1 expression has been shown to be deregulated in vascular and adipose tissue in diabetic animals (3). We assessed GLUT4 trafficking in cardiomyocytes isolated from transgenic (Tg) mice overexpressing SLMAP1 in myocardium. Forced expression of SLMAP1 in cardiomyocytes in vivo resulted in a remarkable increase in vesicle formation with a significant co-localization of SLMAP1 with GLUT4 containing vesicles. A marked expansion in size of GLUT4/SLMAP1 containing vesicles (42.7 fold ± 4.90 fold, n=3, P<0.05) was evident in cardiomyocytes isolated from SLMAP1 transgenic mice compared to wildtype (Wt) littermates. Glycolysis rate measurements were conducted with an XF24e Extracellular Flux Analyzer to assess the extracellular acidification rate (ECAR) upon sequential treatment of cardiomyocytes with glucose, oligomycin, and 2-deoxy-glucose. Basal glycolysis was determined through analysis of ECAR upon addition of glucose and. maximal glycolytic capacity was estimated upon treatment of the cells with glucose and oligomycin. Basal glycolysis in cardiomyocytes from SLMAP1 Tg hearts was increased (317% ± 101%, n=3, P<0.05) compared to those from Wt hearts (165% ± 20.7%, n=3, P<0.05). Maximal glycolytic capacity in cardiomyocytes from Tg hearts was also increased (639% ± 181%, n=3, P<0.05) compared with Wt hearts (365% ± 32.7%, n=3, P<0.05). This data supports a novel role for SLMAP1 in glucose metabolism in the myocardium and suggest that it may be a unique target in metabolic disease.
Table 1. SLMAP1 expression impacts GLUT4 vesicle size in cardiomyocytes
1. Bryant NJ et al (2002). Nat Rev Mol Cell Biol 3: 267–277. 2. Guzzo R et al (2005). Am J Physio Heart Circ Physiol 288: 1810-1819. 3. Ding H et al (2005). Am J Physiol Heart Circ Physiol 289: 206-211 4. Nader M et al (2012). Am J Physio Heart Circ Physiol 302: 1138-1145. 5. Dewan et al (2013). Proceedings of the British Pharmacological Society at http://www.pa2online.org/abstracts/vol11issue3abst216p.pdf
|
||||||||||