Print version
Search Pub Med
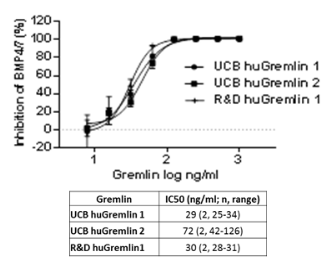
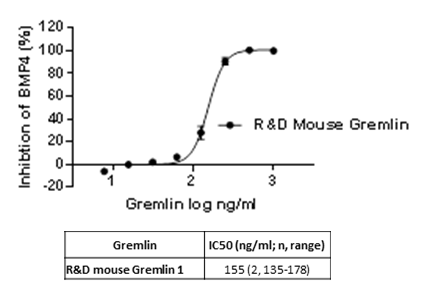
Characterisation and validation of a cellular assay to determine the activity of the BMP antagonising protein, Gremlin Bone Morphogenetic Proteins (BMPs) are members of the TGFbeta superfamily and are multifunctional growth factors regulating a wide range of activities in tissue development and function. Gremlin directly binds and negatively regulates BMP, preventing downstream SMAD signalling. Expression of Gremlin has been shown to be up regulated in various human diseases including idiopathic pulmonary fibrosis (IPF) and kidney fibrosis. Therapeutics able to block the Gremlin/BMP interaction may be of value in these and other fibrotic diseases. We have optimised a series of Gremlin/BMP-dependent cellular Id-1-dependent reporter gene assays using a luciferase reporter cell line. Aim of the Hek Id1 Reporter assay was to assess the blocking activity of human and mouse Gremlin-1 and human Gremlin-2 to rhBMP homodimers 4,7 and heterodimers 2/6, 2/7 and 4/7.We present here the validation and optimisation of the assays and the characterisation of both human and mouse protein reagents from in-house and commercial sources. Hek Id1 reporter cells were plated in a 96 well poly-D-lysine coated plate and incubated for 4 hours at 37°C to adhere. Cells were incubated with Gremlin and rhBMP homodimers or heterodimers for 24hours at 37°C. The Luciferase signal was detected using Steady Glo kit from Promega following the manufacturer’s instructions.
Figure1. A. Concentration response curves of human Gremlin proteins in the Id-1 reporter gene assay activated by heterodimeric BMP-4/7. B, the activity of mouse Gremlin 1 in concentration response against the homodimeric BMP-4-driven assay. Table 1, Assay quality parameters from Gremlin 1-heterodimer BMP-4/7 Id-1 reporter gene assay. Table 1
In summary, Hek Id1 reporter assay has been optimised to achieve improved assay performance. This assay is suitable for characterising the activity of Gremilin-1 and Gremlin-2 as an antagonist of BMP homo- and hetero- dimers.
|