Print version
Search Pub Med
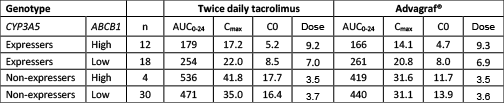
Impact of CYP3A5 and ABCB1 Polymorphisms on the Pharmacokinetics of immediate and prolonged release Tacrolimus Formulations in Renal Transplant Recipients. Background— Tacrolimus is a widely used immunosuppressive drug in organ transplantation with a narrow therapeutic index. It is available in twice daily immediate release formulations and as a once daily prolonged release preparation, Advagraf®. Tacrolimus bioavailability varies greatly between individuals and depends largely on the activity of the CYP3A subfamily and the drug transporter P-glycoprotein (P-gp) encoded by the ABCB1 gene. CYP3A expression decreases and P-gp expression increases along the length of the gut. Objective— The aim of this study was to assess whether the well-defined influence of the CYP3A5*3 and ABCB1 genotypes on the pharmacokinetics of immediate release tacrolimus also applies for the prolonged release preparation. Methods— Sixty-four renal transplant recipients receiving a stable dose of twice daily tacrolimus were switched to the same total daily dose of Advagraf® with 24 hour pharmacokinetic profiles before and two weeks after the change. Patients treated with no more than 5mg prednisolone once daily and enrolled in the study after providing written informed consent. CYP3A5 and ABCB1 genotypes were determined using a Roche LightCycler®. Patients were divided into 4 genotype categories based on expression of CYP3A5 (*1/*1 or *1/*3), CYP3A5 non-expressers (*3/*3), high expressers of P-gp (ABCB1; CC) or low expressers of P-gp (ABCB1; CT or TT). Tacrolimus blood concentrations were measured by liquid chromatography/tandem mass spectrometry and individual pharmacokinetic parameters were analysed using analysis of variance (ANOVA). Results— After categorizing the renal transplant patients in different groups based on their CYP3A5 and ABCB1 genotypes, significant differences in tacrolimus pharmacokinetic parameters were evident between ABCB1 polymorphisms in CYP3A5*1 allele carriers. Dose-adjusted AUC0-24, dose-adjusted Cmax and dose-adjusted trough concentration (C0) were significantly lower in CYP3A5 expressers than in CYP3A5 non-expressers for both preparations (Table). Dose requirement was significantly associated with the combined-genotype grouping. The influence of the CYP3A5 and ABCB1 genotype on tacrolimus exposure was the same for the prolonged release preparation Advagraf® as for the immediate release preparation, Prograf®.
Conclusions— CYP3A5 expression had a major influence and ABCB1 genotype had a minor influence on tacrolimus exposure irrespective of preparation. CYP3A5/ABCB1 combined-genotype approach should be considered for guiding dose selection. Pharmacogenetic dosing strategies based on these genotypes are likely to be equally applicable to prescribing the once daily tacrolimus formulation, Advagraf®, as to twice daily formulations.
|