198P Queen Elizabeth II Conference Centre London
Pharmacology 2014 |
Use of Multi-Criteria Decision Analysis (MCDA) for Assessing the Benefit and Risk of OTC Analgesics.
KD Rainsford2, RA Moore5, RA Pawinski1, LD Phillips4, A Crossely3, O Sancak3, B Ng3. 1PV Medica Ltd., Berkshire, UK, 2Biomedical Research Centre, Sheffield Hallumm University, Sheffield, UK, 3Reckitt Benckiser Consumer Health, Slough, UK, 4London School of Economics, London, UK, 5Pain Research, Nuffield Division of Anaesthetics, University of Oxford, Oxford, UK
Background: EU legislation mandates marketing authorisation holders to perform benefit / risk analysis on their medicines. The European Medicines Agency’s Pharmacovigilance Risk Assessment Committee (PRAC) has recently issued cardiac safety advice for diclofenac based on COX-2 inhibitor effect. This raises questions for prescribers and patients about the benefit and the risk of similar commonly available OTC medicines available in the UK and most EU countries.
 Methods: An expert group applied an MCDA model to assess the benefit / risk balance (1) on six of the most common OTC analgesics; ibuprofen acid, ibuprofen salts & solubilised (S) formulations, paracetamol, diclofenac potassium, aspirin and naproxen sodium. Data were entered into the model corresponding to a preference hierarchy in order of superiority: meta-analyses, clinical trials, registry reviews, product SPCs and clinical judgements. The six drugs were evaluated against 11 criteria: three favourable effects (pain relief, duration of action and speed of onset), and eight unfavourable effects: three common adverse reactions (skin reactions, GI and hepatic), four serious adverse reactions (GI effects, CV effects, renal effects and anaphylaxis), and overdose toxicity. Performance of the drugs was scored on relative 0-to-100 scales for each effect criterion, and weights were assessed to represent the relative clinical relevance of the effect criteria. (see process flow chart) Methods: An expert group applied an MCDA model to assess the benefit / risk balance (1) on six of the most common OTC analgesics; ibuprofen acid, ibuprofen salts & solubilised (S) formulations, paracetamol, diclofenac potassium, aspirin and naproxen sodium. Data were entered into the model corresponding to a preference hierarchy in order of superiority: meta-analyses, clinical trials, registry reviews, product SPCs and clinical judgements. The six drugs were evaluated against 11 criteria: three favourable effects (pain relief, duration of action and speed of onset), and eight unfavourable effects: three common adverse reactions (skin reactions, GI and hepatic), four serious adverse reactions (GI effects, CV effects, renal effects and anaphylaxis), and overdose toxicity. Performance of the drugs was scored on relative 0-to-100 scales for each effect criterion, and weights were assessed to represent the relative clinical relevance of the effect criteria. (see process flow chart)
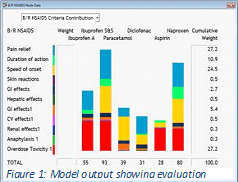
 Results: The overall weighted preference scores (out of a maximum 100) are shown in figure 1. Ibuprofen S, with an overall score of 93, emerged as overall most preferred. Ibuprofen S scored 100 in the model on three of the most heavily weighted effects, safe for overdose toxicity, beneficial for pain relief and speed of onset,. None of the other five drugs did as well. The model was robust over a range of sensitivity analyses confirming that model results would remain relatively unchanged even with significant changes in weights or published evidence. Results: The overall weighted preference scores (out of a maximum 100) are shown in figure 1. Ibuprofen S, with an overall score of 93, emerged as overall most preferred. Ibuprofen S scored 100 in the model on three of the most heavily weighted effects, safe for overdose toxicity, beneficial for pain relief and speed of onset,. None of the other five drugs did as well. The model was robust over a range of sensitivity analyses confirming that model results would remain relatively unchanged even with significant changes in weights or published evidence.
Conclusions: Applying a multi-criteria-decision analysis model to OTC analgesic efficacy and safety data shows an overwhelming preference in benefit / risk in favour of Ibuprofen S. The model provides a robust analysis tool to assist prescribers and patients choose an OTC analgesic for acute pain. The MCDA model confirmed Ibuprofen S has the most beneficial benefit / risk profile of six OTC analgesics. This model approach can be easily adapted to assess other medicines.
References:
1. Nutt D, King LA, et al. (2010). Lancet 376 1558-65
|



