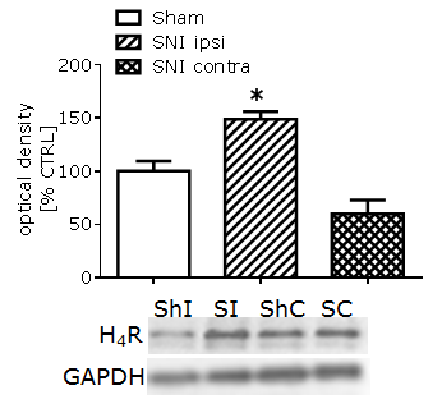
Strain-dependent upregulation of spinal cord H4 histamine receptors in a rodent model of neuropathic pain. There is growing evidence for the role of the histamine H4 receptor (H4R) in sensory neurotransmission and its importance as a novel analgesic target for a range of acute and chronic pain states. However, the lack of commercially available antibodies for H4R significantly limits the majority of these investigations. Thus, in our studies, using our own anti-H4 antibody, we determined the expression of H4R at different levels of sensory nervous system of rats and mice and examined changes in H4R protein level in response to nerve injury that leads to the development of neuropathic pain. Neuropathic pain in mice (Balb/C and CD1, n=4-6) and rats (Wistar, n=4) was induced by speared nerve injury (SNI) or by chronic constriction injury (CCI), respectively. Conventional immunohistochemistry was used to determine expression of H4R in the spinal cord dorsal horn, dorsal root ganglia (DRG), sciatic nerve and skin. Quantitative immunoblotting to examine H4R protein level changes after nerve injury was performed using a validated in house anti-H4 antibody (1 µg/ml). Based on immunofluorescence approaches we have demonstrated for the first time the presence of the H4R in sensory fibres of sciatic nerve and in the skin, and in the neurons of spinal dorsal horn and DRG. Using immunoblotting, we provided preliminary evidence for the upregulation of the rat H4 receptor protein level in the skin, but not the spinal cord dorsal horn, as measured 1 day after nerve constriction in rats. In mice, however, our results indicated that nerve injury (SNI) led to a significant increase (approx. 50%) in the spinal dorsal horn protein level for H4R in Balb/C, but not in CD1 (Figure 1), as measured 7 days after the injury. While Balb/C mice have been shown previously to display a heightened level of innate anxiety compared to CD1, this approach now permits us to investigate the potential relationship between H4R expression, anxiety level and chronic neuropathic pain. Based on our observation, we can hypothesize that this result is a direct response to the injury and/or heightened anxiety, given no significant increase was observed on the side contralateral to the SNI injury. This may potentially increase the pathways in which histamine and H4R are implicated, broadening its neuronal effects. In summary, our results confirm a role for H4R in the regulation and development of neuropathic pain, and show for the first time a potential involvement of H4R in the correlation between neuropathic pain and the level of anxiety. Thus, our data may suggest H4R as a potentially important factor in the regulation of emotional response to pain.
Figure 1. Effect of spared nerve injury (SNI) on the level of H4R in the Balb/C mouse spinal cord dorsal horn, expressed as a percentage of control (sham) and normalized with GAPDH signal. ShI – sham ipsi, SI – SNI ipsi, ShC – sham contra, SC – SNI contra. (*p<0.05) denotes significance vs. sham control (One-way ANOVA, Bonferroni’s post-test). Our studies were funded by Wellcome Trust and RCoA/BJA.
|