Print version
Search Pub Med
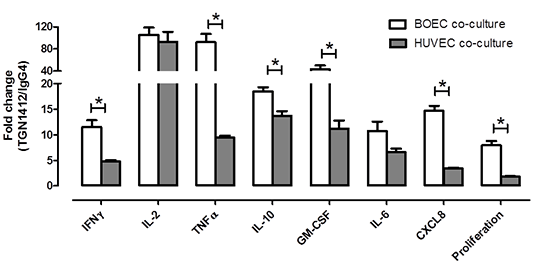
A same donor endothelial cell: PBMC co-culture assay is superior to heterologous cell co-cultures as a cytokine storm response bioassay: relevance to screening biologics for safety The devastating and unpredicted side effects induced by the CD28 superagonist, TGN1412, defined an urgent unmet clinical need for improved human tissue bioassays to detect cytokine storm responses of biological drugs. Current bioassays using peripheral blood monocytes (PBMCs) require an endothelial cell interface or immobilisation of antibody in order to detect TGN1412 or similar drugs. Endothelial cell: PBMC co-culture bioassays however rely on endothelial cells and PBMCs from different donors which we and others(1) suggest could affect sensitivity due to tissue mismatching. In order to address this we devised an assay that uses endothelial cells derived from adult progenitors in the blood (blood outgrowth endothelial cells: BOEC) and thereby allows same donor co-culture of endothelial cells and PBMCs(2). This assay detects cytokine storm responses to the TGN1412-like anti-CD28 superagonist ANC.28. However, ANC.28 is a murine antibody whereas TGN1412 is fully humanised. In order to further validate our assay we have performed new experiments using authentic TGN1412 and compared cytokine responses and cellular proliferation in assays using same donor co-culture of BOEC and PBMCs with the industry standard co-culture assay of mixed donor human umbilical vein endothelial cells (HUVEC) and PBMCs. BOEC(3) and HUVEC(4) were cultured as described previously. Cytokines were measured using ELISA and proliferation by thymidine incorporation(4). PBMCs were isolated and co-cultures prepared according to previous studies4. As we have previously shown neither PBMCs nor endothelial cells responded to TGN1412 when stimulated in mono-culture. However, when co-cultures of either BOEC with same donor PBMCs or HUVEC with heterologous PBMCs were treated with TGN1412 cytokines were released and proliferation induced. When comparing responses to TGN1412 (versus isotype control antibody responses) same donor BOEC : PBMC co-cultures showed a superior signal to noise than when HUVEC were used as an endothelial cell interface (Figure 1).
Figure 1: Comparison of TGN1412 induced responses of BOEC and HUVEC in co-culture with autologous (open bars) or mixed donor PBMCs (filled bars) respectively. Data is presented as fold change of cytokine release or proliferation in cultures stimulated with TGN1412 (1μg/well (3.3μg/ml); 24 hours) compared to IgG4 isotype control. Data are mean ± SEM (n=12-24 from 3 donors). *p<0.05 by t-test. These results confirm that BOECs can be successfully used to construct an autologous co-culture assay with PBMCs that detects cytokine storm responses to TGN1412. Moreover, these results illustrate the utility of this assay by showing that for a number of key readouts it produces a greater signal to noise than the conventional mixed donor HUVEC: PBMC bioassay. 1. Finco D et al. (2014) Cytokine 66:143-155; 2. Reed DM et al. (2013) Toxicology Letters 221 S164-S164; 3. Starke, RD. et al. (2013) Blood 121: 2773-2784; 4. Stebbings R et al (2007) J Immunol 179:3325-31.
|