259P Queen Elizabeth II Conference Centre London
Pharmacology 2014 |
The Pattern of 5-Hydroxytryptamine Receptor Subtypes Mediated Epidermal Growth Factor Receptor Transactivation In Rat Aorta
Sahika Guner1, Tamila Akhayeva2, Hakan Gurdal3. 1Ufuk University, School of Medicine, Department of Medical Pharmacology, Ankara, Turkey, 2Astana Medical University Department of Pharmacology, Astana, Kazakhstan, 3Ankara University, School of Medicine, Department of Medical Pharmacology, Ankara, Turkey
Gqprotein-coupled 5-HT2A and Gi protein-coupled 5HT1B receptor subtypes are mainly found in vascular tissue and they mediate contractile responses in vascular smooth muscle. Epidermal growth factor receptors (EGFR) are transactivated by many G protein-coupled receptors. We have previously shown transactivation of EGFR by α1-adrenergic receptor and 5-HT receptors in vascular smooth muscle(1,2). In this study, we aimed to investigate 5HT2A and 5HT1B receptor mediated EGFR transactivation profile in rat aorta. For this purpose, we examined the effect of the selective 5HT2A agonist α-Methyl-5HT (10μM) and the selective 5HT1B agonist Sumatriptan (Suma, 10μM) on EGFR phosphorylation with and without the EGFR inhibitor AG1478 (10μM) in endothelium-denuded rat aorta. 14-16 week Male-Wistar rats were anesthetized with ketamine/xylasine (100mg/kg,10mg/kg IP), and thoracic aorta were obtained. All animal experiment were performed following approval by Ethics Commitee of Ankara University.Statistical comparison was performed in at least 3 independent experiments using unpaired Student’s t test.Furthermore, we evaluated α-Methyl- 5HT and Suma stimulated auto-phosphorylation (pEGFR1173) and Src kinase-specific phosphorylation (pEGFR845) of EGFR in the presence of Src-kinase or PI-3 kinase inhibitors (PP2, 10µM, LY 294002, 10µM, respectively). Both α-Methyl-5HT and Suma increased pEGFR1173 (Control (C), 100±4,8% vs α-Methyl-5HT; 233±13%; C, 100±32% vs Suma 326±28%) and pEGFR845 (α-Methyl 5HT 166±10%; Suma, 385±15%) compared to unstimulated tissue (n=3). Moreover AG1478 incubation inhibited α-Methyl 5HT and Suma mediated phosphorylation of EGFR (pEGFR1173;α-Methyl-5HT+AG, 90±15%, Suma+AG, 124±15.5% and pEGFR845;α-Methyl-5HT+AG; 70±11% Suma+AG, 153±2.9%). PP2 and LY294002 partially and completely inhibited α-Methyl 5HT-induced phosphorylation of EGFR1173 and EGFR845, respectively.On the other hand, while Suma-induced phosphorylation of EGFR845andEGFR1173 was inhibited by the PI-3 kinase inhibitor, LY 294002, PP2 partly inhibited and did not inhibit phosphorylation of EGFR845 and EGFR1173, respectively. Our results show that Src kinase and PI-3 kinase have similar roles in 5HT2A mediated EGFR transactivation whereas PI-3 kinase has more prominent effect in 5HT1B dependent EGFR transactivation.
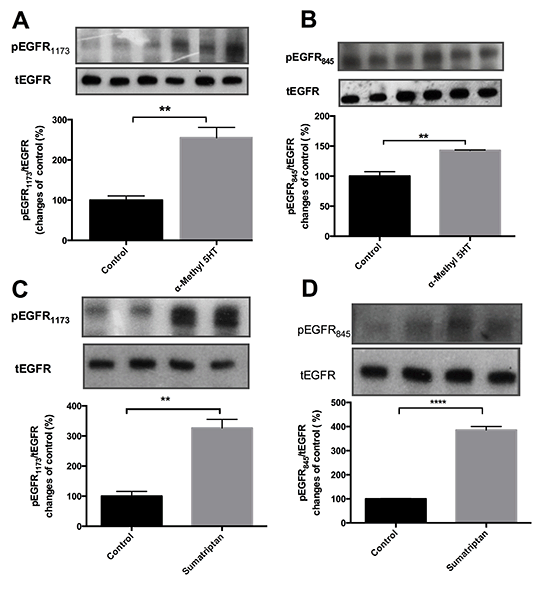
Figure 1. Alpha-Methyl-5HT (10μM, 5 minutes) stimulated A. EGFR1173 and B. EGFR845phosphorylation and Sumatriptan (10μM, 5 minute) stimulated C. EGFR1173 and D. EGFR845 phoshorylation in endotelium-denuded rat throcic aorta.Net intensities of pEGFR and tEGFR blots were calculated three separated experiments. Bar graphs were represented ratio of pEGFR-to tEGFR compared to control (unstimulated tissue). Data were shown as a (Mean ± S.E.M). Statistical differences **P< 0,01 ****P< 0,0001 vsC group.
1. Ulu N et al (2013). The Journal of Pharmacology and Experimental Therapeutics 347(1)47-56
2. Guner S et al (2014). The FASEB Journal (28 no. 1 Supplement 1065.7)
|