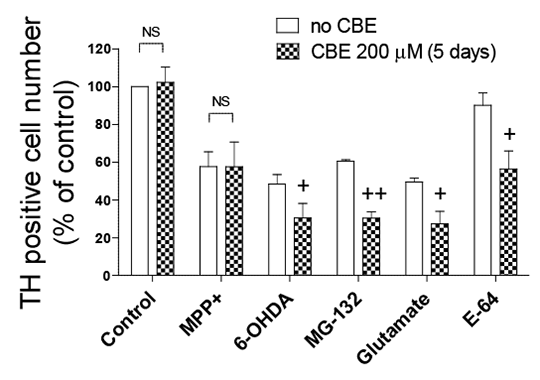
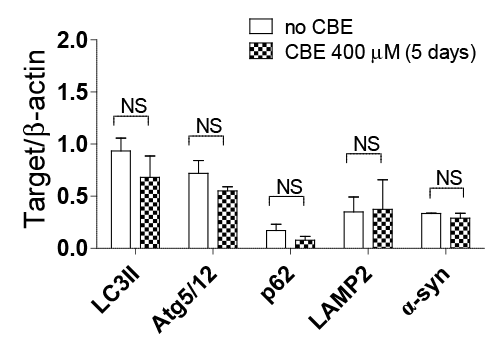
Inhibition of glucocerebrosidase potentiates toxin-induced dopaminergic cell loss in rat ventral mesencephalic cultures Pathogenic mechanisms responsible for dopaminergic cell death in Parkinson’s disease (PD) include mitochondrial dysfunction, oxidative stress, dysfunction of the proteasome system, and impaired lysosomal function. Mutations in the lysosomal enzyme glucocerebrosidase (GBA) gene causing Gaucher’s disease result in a markedly increased risk for developing PD (1). Because most GBA mutation carriers, even homozygotes, do not develop PD, GBA mutations are most likely to be a susceptibility factor for PD, through an interaction with other cell death mechanisms. This study investigated the impact of inhibition of GBA on dopaminergic cell death in primary cultures induced by a range of toxins via alteration of mitochondrial and proteasomal function and induction of oxidative stress. Dopaminergic cell viability was assessed by immunocytochemistry in rat embryonic ventral mesencephalic (VM) culture exposed for 3 h to MPP+ (25 µM), 6-OHDA (45 µM), MG-132 (0.2 µM), glutamate 10 mM), or the lysosomal inhibitor, E-64 (10 µM), following a 5 day incubation with the pharmacologic inhibitor of GBA, conduritol B epoxide (CBE; 200 µM). The effect of CBE (400 µM) on the levels of proteins involved in the lysosomal degradation pathway (ie. LC3II, Atg 5/12, LAMP2 and p62) and α-synuclein in SH-SY5Y cells was also explored using Western blotting. Data are presented as mean ± SEM. Statistical analysis was performed by two-way ANOVA with a Newman Keuls post-hoc test or paired Student’s t-test where appropriate.
Figure 1: Effect of CBE on cell death in VM cell culture. +P<0.05, ++P<0.01 vs. absence of CBE
Figure 2: Effect of CBE on autophagy markers and α-synuclein using Western blot In rat VM culture GBA inhibition by CBE (200 µM) resulted in enhanced sensitivity to all the neurotoxins and E-64, except MPP+ (Fig. 1) (n=3). CBE (400 µM) did not alter the levels of LC3II, Atg 5/12, LAMP2, p62 or α-synuclein in SH-SY5Y cells following GBA inhibition (Fig. 2) (n=3). Pharmacological inhibition of GBA with CBE did not compromise any enzymes in the lysosomal degradation pathway but contributed to dopaminergic cell death via an interaction with toxins known to induce cell death via mechanisms involved in PD. These results agree with previous reports (2,3) and suggest that mutations in GBA may increase susceptibility to exogenous or endogenous toxins through impaired lysosomal function. (1) Rosenbloom B et al (2011). Blood Cells Mol Dis 46: 95-102. (2) Dermentzaki G et al. (2013). PLoS One 8: e60674. (3) Pelled D et al (2000). J Inherit Metab Dis 23: 175-184. |