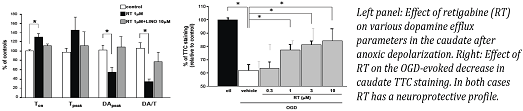
Role Of Kv7 Channels In Anoxia-Induced Damage In Brain Slices Ischaemic stroke causes the activation of multiple processes, including ionic imbalance, peri-infarct depolarization and excessive neurotransmitter release [1]. Although glutamate release has been viewed as a main cause of neuronal damage, massive release of dopamine (DA) also occurs, directly contributing to cell death [2]. Voltage-gated Kv7 potassium channels family comprises five members (Kv7.1- Kv7.5) that have been demonstrated to regulate DA release in the caudate, a region rich in DA and frequently damaged in ischemic stroke [3]. In this study we investigated the potential neuroprotective role of Kv7 channels in an in vitro model of ischaemia, namely rat brain slices undergoing oxygen- and glucose- deprivation (OGD). In particular, we evaluated the effects of different Kv7-acting drugs on: 1) OGD-induced DA release, measured by fast cyclic voltammetry [4]; 2) OGD-induced damage, assessed by 2,3,5-triphenyltetrazolium chloride (TTC) staining. Values are means ± S.E.M., expressed as percentage of controls. The Kv7 activator retigabine (RT) 1 μM increased time to onset (T-on) of OGD-induced DA release (131 ± 7), and also reduced DA peak (DApeak) and rate of change of DA efflux (DA/Tpeak) when compared to vehicle-treated slices (DApeak 57 ± 12; DA/Tpeak 40 ± 9). RT (0.3 to 10 μM) dose-dependently reduced the loss of TTC staining induced by OGD. Kv7.2/Kv7.3-preferring activator ICA27243 1 μM decreased DA peak (43 ± 10) while it did not significantly affect T-on (102 ± 19) and DA/Tpeak (121 ± 35). Moreover, ICA27243 (0.1 to 10 μM) dose-dependently prevented the loss of TTC staining in brain slices exposed to OGD. By contrast Kv7 activator NS15370 0.1 μM almost doubled time to reach DA peak (200 ± 34), but it showed no significant effects on other parameters. Among Kv7 blockers, while XE991 did not interfere with DA release from caudate, linopiridine 10 μM increased DApeak (418 ± 85) and DA/Tpeak (436 ± 75). In addition, when incubated with RT 1μM, linopiridine 10 μM prevented the changes in voltammetric parameters induced by RT after OGD superfusion. Exposure of brain slices to linopiridine (3 to 30 μM) did not have any effect at lower doses, but enhanced OGD-induced loss of TTC staining at the higher concentration tested by Ì´20%. Moreover, linopiridine 10 μM reversed the reduction in loss of TTC-staining exerted by RT 1 μM. Quantitative-PCR experiments showed a Ì´6-fold decrease in Kv7.2 transcript, while levels of mRNAs encoding for other Kv7 subunits were unaffected; western blot experiments showed a parallel reduction in Kv7.2 protein levels.
Taken together, these results suggest a main role for Kv7.2 also in the modulation of DA release induced by an ischaemic insult and highlight pharmacological activation of Kv7 channels as a possible strategy for the treatment of brain ischaemia. 1. Doyle et al., (2008); Neuropharm 55:310-8; 2. Lieb et al., (1995) Exp Neurol 134:222-9; 3. Martire et al., (2007) J Neurochem 102:179-93; 4. Davidson et al., (2011) J Neuro Meth 202:165-72.
|