284P Queen Elizabeth II Conference Centre London
Pharmacology 2014 |
Nitrergic dysfunction in the isolated distal ileum preparation of MPTP treated common marmoset (Callithrix jacchus).
J Dolan2, A Gant2, MJ Jackson1, S Rose1, KR Chaudhuri1, P Jenner1, MM Iravani2,1. 1 King's College London, London, UK, 2University of Hertfordshire, Hatfield, UK.
Impairment of bowel function characterized by decreased frequency of bowel movements, or constipation is the most widely recognized non-motor symptom in Parkinson’s disease (PD) (1). The cause of this gastrointestinal (GI) dysfunction in PD is not fully known but experimental evidence in primates suggest that loss of central dopaminergic system may lead to plasticity in the expression and function of various enteric neurotransmitters (2). It is generally believed that the main contractile neurotransmitter in the GI tract is acetylcholine (ACh) while nitric oxide (NO) causes relaxation of smooth muscle in addition to modulating acetylcholine release in response to electrical field stimulation (EFS) (3).
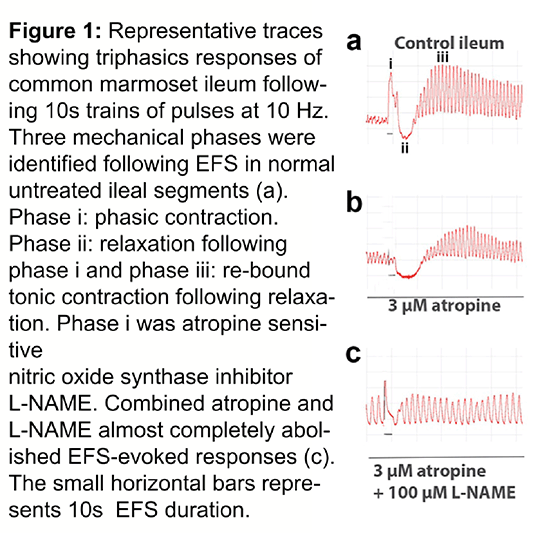
 In this study we compared the contractile / relaxation profile of the responses of distal ileum isolated from male and female normal, drug naïve (n=6) and previously MPTP treated (n=6; 2mg/kg/day, s.c. for 5 consecutive days) common marmosets following pentobarbital euthanasia (60 mg/kg). Ileal segments, 3 cm long were mounted in 25 ml organ baths containing 95% O2 / 5% CO2 aerated Krebs solution at 37°C, straddled between a pair of platinum stimulating electrodes under 1.0 g tension. The tissues were stimulated by 10s trains of pulses, 0.2 ms pulse-width delivered at frequencies ranging from 1, 2, 5, 10, 20, 40 and 60 Hz in the absence or in the presence of 3µM atropine or L-NAME at concentrations ranging from 0.01-1 mM. Changes in tissue tension were recorded using an isometric transducer connected to a digital chart recorder. Following 10s EFS, the normal ileum produced a triphasic response profile as shown in figure 1. Phase i tension increased frequency dependently from 1 Hz (0.61 ± 0.31g) to 20 Hz (0.86 ± 0.22g) but at 40 and 60Hz no further increase was observed. In MPTP treated tissues however, significantly larger contractions were observed beyond 10Hz: at 20 Hz, 1.99 ± 0.78g, at 40 Hz: 2.73 ± 1.12g; P=0.0049; F (1, 70) = 8.439, 2-way ANOVA. By contrast, phase ii responses were absent in MPTP treated preparations at all frequencies tested; P<0.0001, F (1, 70) = 50.28, 2-way ANOVA. However there was no significant difference in the phase iii responses between the normal and MPTP tissues (P=0.672; F (1, 70) = 0.1809). L-NAME at concentrations 0.1 and 1 mM significantly enhanced the phase i contractions at frequencies larger than 20 Hz by up to 250%; P=0.0004; F (3, 140) = 6.396. At all concentrations used, L-NAME significantly blocked the phase ii relaxations P=0.0003; F (3, 140) = 6.807. However, in the MPTP tissues, L-NAME had no significant effect on either of the EFS-evoked responses. In summary, enhancement of phase i and inhibition of relaxation phase ii by L-NAME is line with NO’s direct relaxing effect on smooth muscle and its indirect inhibitory effect on ACh release (3). Greater magnitude of phase i, lack of phase ii and inefficacy of L-NAME in MPTP treated tissues suggests that dopaminergic loss may lead to dysregulation of nitrergic systems in the GI tract in PD. In this study we compared the contractile / relaxation profile of the responses of distal ileum isolated from male and female normal, drug naïve (n=6) and previously MPTP treated (n=6; 2mg/kg/day, s.c. for 5 consecutive days) common marmosets following pentobarbital euthanasia (60 mg/kg). Ileal segments, 3 cm long were mounted in 25 ml organ baths containing 95% O2 / 5% CO2 aerated Krebs solution at 37°C, straddled between a pair of platinum stimulating electrodes under 1.0 g tension. The tissues were stimulated by 10s trains of pulses, 0.2 ms pulse-width delivered at frequencies ranging from 1, 2, 5, 10, 20, 40 and 60 Hz in the absence or in the presence of 3µM atropine or L-NAME at concentrations ranging from 0.01-1 mM. Changes in tissue tension were recorded using an isometric transducer connected to a digital chart recorder. Following 10s EFS, the normal ileum produced a triphasic response profile as shown in figure 1. Phase i tension increased frequency dependently from 1 Hz (0.61 ± 0.31g) to 20 Hz (0.86 ± 0.22g) but at 40 and 60Hz no further increase was observed. In MPTP treated tissues however, significantly larger contractions were observed beyond 10Hz: at 20 Hz, 1.99 ± 0.78g, at 40 Hz: 2.73 ± 1.12g; P=0.0049; F (1, 70) = 8.439, 2-way ANOVA. By contrast, phase ii responses were absent in MPTP treated preparations at all frequencies tested; P<0.0001, F (1, 70) = 50.28, 2-way ANOVA. However there was no significant difference in the phase iii responses between the normal and MPTP tissues (P=0.672; F (1, 70) = 0.1809). L-NAME at concentrations 0.1 and 1 mM significantly enhanced the phase i contractions at frequencies larger than 20 Hz by up to 250%; P=0.0004; F (3, 140) = 6.396. At all concentrations used, L-NAME significantly blocked the phase ii relaxations P=0.0003; F (3, 140) = 6.807. However, in the MPTP tissues, L-NAME had no significant effect on either of the EFS-evoked responses. In summary, enhancement of phase i and inhibition of relaxation phase ii by L-NAME is line with NO’s direct relaxing effect on smooth muscle and its indirect inhibitory effect on ACh release (3). Greater magnitude of phase i, lack of phase ii and inefficacy of L-NAME in MPTP treated tissues suggests that dopaminergic loss may lead to dysregulation of nitrergic systems in the GI tract in PD.
1. Chaudhuri KR, et al. (2006). Lancet Neurol. 5(3): 235-245.
2. Chaumette T, et al., (2009). Neurogastro. Motility 21(2): 215-222.
3. Hebeiss K, Kilbinger H (1998). Neuroscience 82(2): 623-629.
|


