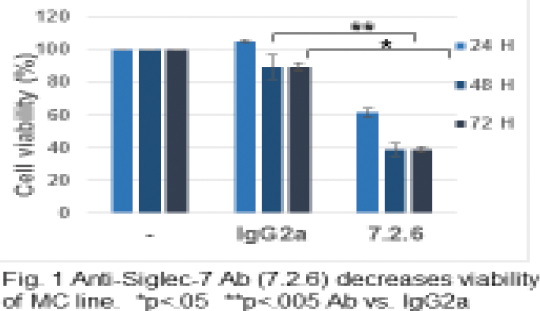
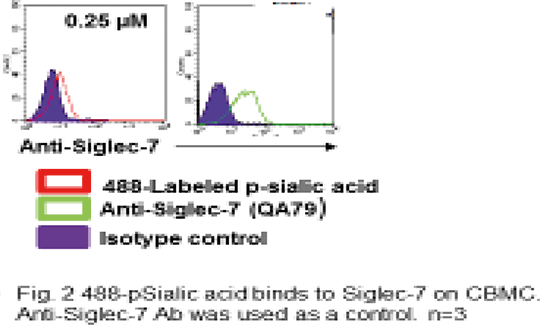
Siglec-7 as a Targetable Receptor on Mast Cells Mast cells (MC) and eosinophils (Eos) are the pivotal cells of allergic inflammation(AI) also by their cross-talk taking place though a plethora of soluble mediators and physical ligands/ receptors interactions that enhance the functions of both the cells. Recently it has become evident that MC and Eos can be regulated by signals other than the allergic classical ones, such as the ones through CD2 family activating receptors(AR) and Ig family inhibitory receptors (IR). Siglecs are a family of sialic acid-binding immunoglobulin like lectins, broadly expressed in human and rodent systems that regulate the functions of cells in the innate and adaptive immune systems through glycan recognition. Siglec-7 (sialic acid binding Ig-like lectin-7), also named p75/AIRM1 and CD328, is an IR, expressed by human natural killer (NK) cells as well as other immune cells that we have recently described in MC [1]. After engagement of the receptor, preferentially through α2,8-disialyl and branched α2,6-sialyl carbohydrate structures [2] the association of its immune-receptor Tyrosine-based inhibitory motif (ITIM) on its cytoplasmic domain with the inhibitory phosphatase SHP-1 occurs [3] resulting in inhibitory signals. The function of IR has been previously shown to be improved when the receptor itself is co-cross-linked to the IgE receptor FcεRI. Specifically, after engagement of Siglec-7 using monoclonal antibody (mAb), it significantly inhibited FcεRI-dependent human cord blood MC (CBMC) activation and the release of GM-CSF, tryptase, beta-hexosaminidase (beta-hex) and the de novo-synthesized lipid mediator PGD2 [1]. In this study we aim to demonstrate that cross-linking of the receptor to itself inhibits transformed MC survival in a time dependent fashion (Fig 1) and to show that synthetic glycopolymers (488 labeled pSialic acid) [4] (kind gift of Prof. Carolyn Bertozzi, U of Calif at Berkeley) (recently used as functional mimics of cell-associated glycans), in addition to the mAb, bind to the IR on CBMC (Fig 2). Human MC leukemic line (HMC-1.1) was incubated with anti-Siglec-7 mAbs and cell viability was measured at different time points (24, 48, 72hrs) by trypan blue exclusion test and thiazolyl Blue Tetrazolium Blue(MTT) assay. Cell viability significantly decreased after 48 and 72h incubation with anti-Siglec-7 mAb. Furthermore, 488 labeled p-sialic acid bound to CBMC indicating that the natural ligand could also induce the effects we describe with the Abs. These results shed light on the importance of Siglec-7 IR as a targetable receptor in various processes such as MC leukemia, allergy and other inflammatory conditions in which MC have a role.
1. Mizrahi, S et al (2014). J Allergy Clin Immunol 134(1): 230-3. 2. Yamaji, T et al.(2002). J Biol Chem 277(8): 6324-32. 3. Falco, M et al. (1999). J Exp Med 190(6): 793-802. 4. Hudak, J.E et al. (2014). Nat Chem Biol 10(1): 69-75.
|