Galanin Receptor Dimerisation Investigated by the BRET Technique
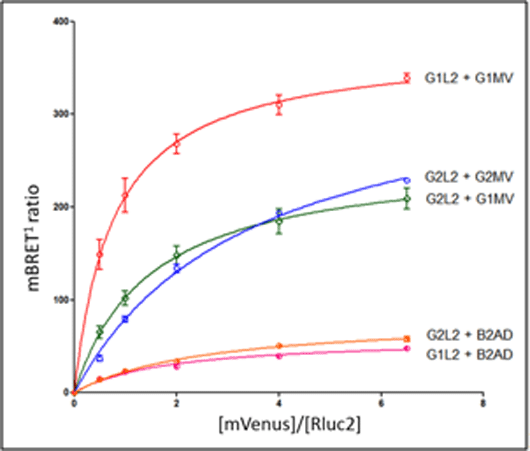
Galanin is a 29 (30 in humans) amino acid regulatory peptide, which is expressed widely in the CNS and in the periphery. Galaninproduces its effects through three Gprotein-coupled receptors; GalR1, GalR2 and GalR3 [1, 2]. Galanin is involved in nociception and neuropathic pain, and both GalR1 and GalR2 are expressed in Dorsal Root Ganglia (DRG) neurons in the spinal cord.At higher doses galanin producesantinociceptive effects, whereas at lower dose it can bepronociceptive.It is not yet clearly understood how galanin receptors achieve these effects. Possible heterodimerisationbetween co-expressed GalR1 and GalR2 receptors may modulate receptor signalling and help to explain some of the effects of galaninin vivo [3]. The aim of this study was to use GalR1 and GalR2 constructs to study potential dimerisation between these two receptors by the Bioluminescence Resonance Energy Transfer (BRET) technique. The galanin receptor constructs (Table 1) were cloned and sequenced. Confocal imaging studies in ND7/23 (mouse neuroblastoma x rat DRG hybrid) cells transiently expressing the constructs demonstrated that all of the receptor constructs areexpressed at the cell membrane. Construct functionality was confirmed by internalization and Arrestin-3 recruitment assays.In the BRET saturation assay, a constant amount of donor (-Rluc2), and increasing amounts of acceptor (-mono Venus) constructs were transfected. The results obtained (Figure 1) suggested that GalR1 or GalR2 form homodimers, as well as GalR1/GalR2 heterodimers. The agonist galanin did not appear to influence GalRdimer formation.In BRET competition assays,a constant amount of donor and acceptor at a ratio of 1:1, and increasing amounts of potentially competing non-donor/non-acceptor GalRwere expressed.The ability of the non-donor/acceptor GalR constructs to decrease the BRET signal supports the idea of homodimerisation and heterodimerisation of GalR1/2 in the ND7/23 cells.
Table 1. DNA constructs for BRET
Figure 1. BRET saturation assay In summary, BRET saturation experiments as well as BRET competition assays suggest that both GalR1 and GalR2 form homodimers, as well as GalR1/GalR2 heterodimers, but in an agonist-independent manner. Currently, experiments are being performed to investigate the effect of potential dimer formation on GalR trafficking and signalling. [1] Fathiet al. (1997). Molecular Brain Research, 51, 49-59. [2] Habert-Ortoliet al. (1994). Proc Natl AcadSci U S A, 91, 9780-9783. [3] Burgoet al. (2010). Expert Opin Drug Discov, 5, 461-74.
|