Pharmacological characterization of the glucagon receptor in a hepatocyte cell line; Hep 3B
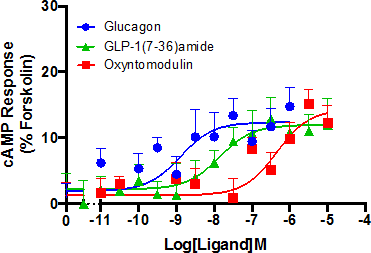
The glucagon receptor (GCGR), a family B G protein-coupled receptor (GPCR), plays an important role in regulating blood glucose levels through its ability to bind the 29 amino acid peptide hormone glucagon. This leads to the stimulation of glycogenolysis and gluconeogenesis in the liver, effectively counteracting the consequences of excessive insulin [1]. Primary human hepatocytes are considered to be the gold standard for the study of metabolism in the liver but their phenotypic instability and restricted accessibility has made way for alternative such as the immortalized hepatocellular cell line, Hep 3B. Despite the use of this and similar cell lines in research, little is currently known about the receptor expression and associated pharmacology. This work aims to give an insight into this with a particular focus on GCGR. Using both qRT-PCR and the secondary messenger cAMP accumulation assay, it was shown that Hep 3B cells express GCGR. Glucagon, GLP-1(7-36)amide (passage number <8) and oxyntomodulin showed activity at the GCGR (pEC50 of 8.90 ±0.38, 7.99 ±0.45 and 6.32 ±0.33 respectively) (Figure1), irrespective of the presence of the GLP-1 receptor (GLP-1R) antagonist exendin(9-39)amide. A pA2 value of 6.5 ±0.25 was determined for the GCGR antagonist des-His1[Glu9]-glucagon amide in Hep 3B cells but showed no activity at the GLP-1R expressing cell line stimulated with GLP-1(7-36)amide. Combined, these findings confirming the activity of glucagon in Hep 3B cells is indeed through the GCGR and not through GLP-1R. Although the functional coupling of a single GPCR to more than one G protein has been known for years, it is a relatively new phenomenon that ligands can determine a bias towards a particular G protein or β-arrestin [2]. Due to the central role in the regulation of blood glucose, expanding the current knowledge available on the GCGR is of huge academic and potentially therapeutic importance. Following on from our establishment of Hep 3B as a suitable cell line for the study of endogenously expressed GCGR, we now aim to investigate alternative G proteins coupling and β-arrestin recruitment following stimulation with a number of ligands active at the GCGR.
Figure 1: Signaling profile of Hep 3B cells. cAMP accumulation was detected after exposure to agonist for 30 min. Responses were normalized to the forskolin response, which was used to determined the maximal possible response of the system. All values are mean ± SEM were n = 5 independent experimental repeats.
References [1] Campbell JE, Drucker DJ (2015). Nat Rev Endocrinol 11: 329–338; [2] Lefkowitz RJ, Shenoy SK (2005). Science 308: 512–517.
|