Development and validation of a Rhizopusoryzae infection model in the mouse
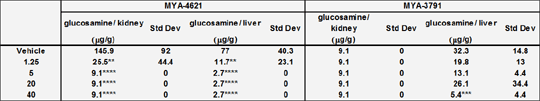
Mucormycosis,is a disease resulting in significant morbidity and mortality with Rhizopusoryzae being one of the most frequently isolated causative agents. The development of novel therapies for such diseases is often hampered by a lack of a simple animal model. The purpose of this study was to develop a murine model of mucormycosis that demonstrated a consistent and robust infection, allowing further study of disease progression and the evaluation of novel therapeutic candidates. R. oryzae strains ATCC MYA-4621 and MYA-3791 were grown over 7 days on potato dextrose agar (PDA) slants at 37°C. Female Balb/c mice (14-22 g, n=8/group) were infected with either 5x104 or 5x105 cfu/mouse by intravenous injection. Animals were observed, and removed from study by euthanasia with CO2 asphyxiation if there was deterioration in clinical condition. Tissues were analysed for fungal burden using methods from Rementeriaet al (1). Once the optimum infecting dose was established, the model was validated with Liposomal Amphotericin B (LAmB) dissolved in DI water. This was administered daily by intraperitoneal injection at doses ranging from 0.16 – 40 mg/kg/day. Survival data was analysed using A Kaplan Meier survival plot and Kruskal-Wallis ANOVA followed by a Dunn’s multiple comparison test were used for fungal burden analysis, with P<0.05 taken to be significant. Data were expressed as mean ± standard deviation. In the absence of treatment, the median survival time was 1-2 days depending on the strain used for infection. Treatment with LAmB had a significant improvement on survival time at doses of 5mg/kg and above with the median survival time at 20 mg/kg being 7 and 4 days for MYA-4621 and MYA-3791 respectively. There was no additional benefit from increasing the dose to 40 mg/kg. Fungal burden was also significantly reduced in both strains in the kidney and liver when levels in LAmB treated animals were compared to vehicle treated control animals (Table1)
Table 1 : Fungal burden in mice infected with MYA4621 and MYA-3791 and treated with LAmB or vehicle. ** P<0.01, *** P<0.001, ****P<0.0001 versus vehicle control
This systemic infection model can now be used to characterize novel therapies for the treatment of mucormycosis. (1) Rementeria et al (1991). Journ Med Vet Mycology. 29:15-23.
|