Ouabain Causes Temperature-Dependent Action Potential Decline In Ex Vivo Optic Nerve
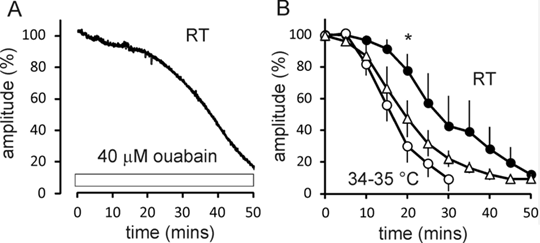
Membrane properties of optic nerve axons exhibit a marked temperature dependence, including dramatically altered refractory periods (1). This may be explained by the presence of a substantial electrogenic component of the membrane potential and a temperature dependent Na+ influx (1), via electroneutral ion transporters and exchangers, whose activity is known to increase with warming (e.g. 2). Optic nerve was isolated from C57bl/6 mice (male and female) which were >10 weeks old, and culled in accordance with Home office guidelines. The nerves were mounted in an ex vivo bath and stimulated using constant current pulses via a suction electrode (0.6 Hz). The action potential was recorded across a petroleum jelly barrier. The bath was continuously perfused with oxygenated buffer solution and the temperature constantly monitored. We have measured the supramaximal compound action potential amplitude in mouse optic nerve as the electrochemical gradient for Na+ collapses on exposure to 40 µM ouabain (Fig 1A). By partially blocking the Na+ pump, the action potential amplitude fell away over 10s of minutes, and the rate at which the action potential declined was significantly increased by warming from room temperature (RT) to 34-35 °C (p=0.014; n = 4, 5, respectively; t-test at 20 mins, *), consistent with increased Na+ entry at the higher temperature (Fig 1B). The decline in amplitude at 34-35 °C appears slowed by exposure to the Na+ transport blocker bumetanide (500 nM- 5 µM), and in the presence of the drug the reduced action potential was maintained at 50 mins (p=0.041, n=5, one-sided t-test; Fig 1B)
Figure 1. A) Typical data-set showing the action potential amplitude in mouse optic nerve at RT decline on exposure to 40 µM ouabain (applied during open bar). B) Mean RT data (filled circles). Raising the temperature to 34-35 °C significantly increased the rate of decline (open circles; * p=0.014). Bumetanide (applied from time 0) at 34-35 °C (open triangles) on average slowed the decline, although this was not-significant after 20 mins. Data plotted as means ± s.e.m.
These findings are consistent with a temperature-dependent Na+ entry into central axons (1), that is only partly blocked by bumetanide. We hypothesize that block of such Na+ entry may reduce axonal energy expenditure and confer protection in pathological circumstances where metabolism is compromised. We acknowledge the support of the MS society (1). Coates, TA et al Pflugers Arch. DOI 10.1007/s00424-015-1696-2(2015). (2). Zeuthen, T and MacAuley, N JPhysiol. 590: 1139(2012)
|