Print version
Search Pub Med
Oscillatory Phasic Activity in Human Pulmonary Veins Could be a Unique Model for Atrial Fibrillation
Introduction Ectopic activity in the pulmonary vein myocardial sleeves is known to play an important role in the pathogenesis of atrial fibrillation (AF). Oxidative stress in the pulmonary vasculature, which can occur during cardiothoracic surgery and sleep apnoea, is associated with an increased incidence of AF. This study investigated the effects of hypoxia and reoxygenation on human pulmonary vascular tone. In isolated human pulmonary veins a serendipitous observation was suggestive of automaticity and we hypothesise that the human pulmonary vein preparation could be a simple and effective model for the study of AF and to investigate the efficacy of novel antiarrhythmic agents. Methods Rings of normal human pulmonary arteries and veins were obtained at resection for cancer and mounted between stainless steel wires in 25 ml organ baths (containing Krebs-Henseleit solution, bubbled with 95% O2: 5% CO2) to measure changes in isometric tension. The effects of hypoxia on resting and active tension were determined by changing the aerating gas to 95% N2: 5% CO2.
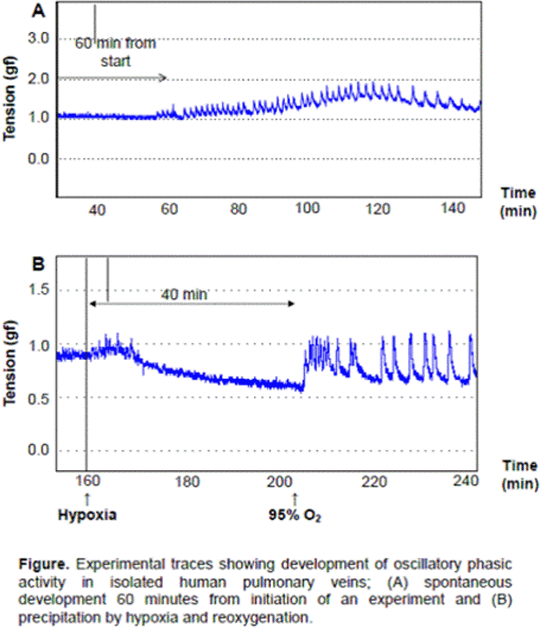
Results Seventeen rings of human pulmonary vein were obtained from seven patients (mean internal diameter 4.9 ± 0.78 mm, 3.7 ± 0.27 mm long). The pulmonary veins were found to exhibit an oscillatory phasic activity which occurred spontaneously in eleven veins after 49 ± 6 minutes (Figure A) or was precipitated by exposure to hypoxia and reoxygenation in four veins (Figure B). Two veins did not exhibit phasic activity. Phasic activity was not observed in isolated human pulmonary arteries (n > 40) and may originate from automaticity in the pulmonary vein myocytes and could therefore be arrhythmogenic in nature. Conclusion In conclusion the isolated human pulmonary vein preparation could be a simple and effective model for investigating the pathogenesis of arrhythmic activity originating in the human pulmonary venous system and could facilitate the rapid screening of novel antiarrhythmic agents.
|