Worldwide withdrawal of medicinal products because of adverse drug reactions: an analysis of withdrawal patterns
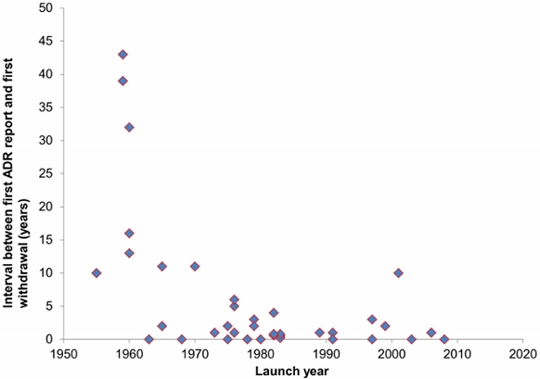
Introduction: We have systematically identified medicinal products withdrawn worldwide because of adverse drug reactions, assessed the level of evidence used for making the withdrawal decisions, and explored the patterns of withdrawals over time. Methods: We searched PubMed, the WHO database of withdrawn products, and selected texts, as previously described (BMC Medicine 2015; 13: 26). We also hand searched references of retrieved documents. We included products that were withdrawn after launch from 1950 onwards, excluding non-human and over-the-counter medicines. We assessed the levels of evidence on which withdrawals were based using the Oxford Centre for Evidence Based Medicine Levels of Evidence (www.cebm.net). Results: Of 347 medicinal products withdrawn from any country because of adverse drug reactions, only 40 were withdrawn worldwide. Anecdotal reports were cited as evidence for withdrawal in 30 (75%) and deaths occurred in 24 (60%). Hepatic, cardiac, and nervous system toxicity accounted for over 60% of withdrawals. In 27 cases, withdrawal was initiated by the manufacturer. The median interval between the first report of an adverse drug reaction and the first withdrawal was 1 year (range 0 to 43 years). The interval between reports of adverse drug reactions and worldwide withdrawal shortened over time (Figure 1); in all these cases worldwide withdrawal occurred within 1 year after the first withdrawal in any country. Conclusion: The time it takes for drugs to be withdrawn worldwide after reports of adverse drug reactions has shortened over time. However, the speed with which worldwide withdrawals occurred after initial withdrawal suggests possible selective reporting of potentially serious harms in the pre-approval phase. There are inconsistencies in current withdrawal procedures when adverse drug reactions are suspected. A uniform method for establishing worldwide withdrawal of approved medicinal products when adverse drug reactions are suspected should be developed. Improved transparency and coordination of harms reporting in clinical trials is imperative, in order to facilitate global withdrawals.
Figure 1: The interval between the first report of an adverse drug reaction (ADR) and the first withdrawal of a medicinal product withdrawn worldwide because of that ADR versus launch year
|