Use of fixed dose combination (FDC) antibiotics in India: an examination of regulatory approvals, sales volumes, and manufacture by non-Indian multi-national companies in the context of anti-microbial resistance concerns
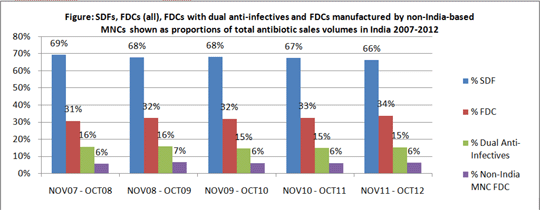
Antibiotic resistance is of increasing concern globally but initiatives to curtail inappropriate consumption have had little success. During 2000-10, sales of antibiotics rose by 36% internationally, including of ‘last resort’ agents and especially in India (1). Investigations have focused on individual antibiotics, overlooking other potentially relevant factors including the use of fixed dose combinations (FDCs), formulations comprised of two or more drugs combined in a fixed ratio of doses and available in a single dosage form. FDCs with more than one anti-infective drug are generally unsuitable for treating bacterial infection, tuberculosis being an exception. In India, FDCs are used extensively for many indications despite disquiet about their regulation and safety (2). There has been little study of antibiotic FDC use. We investigated systemic (oral and parenteral) antibiotic use in India by undertaking a time-trend analysis of antibiotic sales over five years, 2007-2012. We determined the proportions of sales comprised of single drug formulations (SDFs) and of FDCs, the regulatory approval status of marketed FDCs, and the contribution made to sales by FDCs manufactured by non-Indian multi-national companies (MNCs). We obtained information on approvals from the national regulator, the Central Drugs Standard Control Organisation (CDSCO), and on sales from PharmaTrac, a commercial database of national drug sales, formulations, products marketed and their manufacturers. There were 118antibiotic FDC formulations and 86 SDFs on the market; 38/118 FDCs (32%) had a record of CDSCO approval; 80/118 (68%) had no record. Total antibiotic sales volumes rose by 26% during 2007-2012 (from 2055m Units to 2582m). FDC proportions increased annually (Figure); 55/118 FDCs (47%) included two anti-infectives of which 35/55 were two antibiotics. Dual anti-infectives accounted for 15% of total sales volumes. Non-India MNCs manufactured 6% of the total (Figure). Of the 20 top-selling antibiotic FDCs, only 9/20 had a record of CDSCO approval of which 3 were FDCs also approved by US and EU regulators; 18/20 were manufactured by non-Indian MNCs. We conclude that antibiotic FDC use in India is substantial, many FDCs have no record of CDSCO approval and many are manufactured by non-Indian MNCs as well as by Indian manufacturers. In the context of resistance concerns, antibiotic FDC regulation and use in India merit scrutiny.
1. Van Boekel T et al. (2014) Lancet Infect Dis 14: 742–50. 2. McGettigan P et al. (2015) PLoS Med 12(5): e1001826
|