Print version
Search Pub Med
Using esterase sensitivity to limit the systemic availability of topical medicines – an example with β-blockers.
Topical β-blockers are used in glaucoma and increasingly for infantile haemangioma (Léauté-Labrèze et al., 2008). However, significant systemic absorption occurs causing low blood pressure and bradycardias and there is concern about the developmental effects of chronic β-blockade in babies (Filippi et al., 2013). A topical agent, devoid of any systemic availability would be beneficial. We synthesised a series of novel ester β-blockers, measured their β-blocking effects and esterase sensitivity using 3H-CGP 12177 whole cell binding (CHO-β1 cells) and monitored fenoterol-stimulated heart rate following intravenous (iv) injection in conscious Sprague-Dawley rats (Baker et al., 2011).
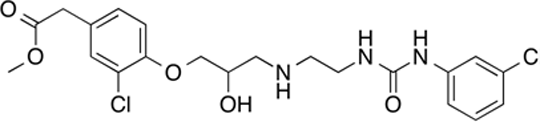
NDD-446
The binding of betaxolol to the β1-adrenoceptor (log KD-8.06 ± 0.03, n=15) was not affected by a 6hr co-incubation with liver esterase (0.85u/ml, rightward log shift of competition binding curve = 0.01 ± 0.02, n=11) orpurified human serum butylylcholinesterase(0.79u/ml, log shift of 0.01 ± 0.01, n=4). When injected iv into conscious rats, betaxolol0.1mg/kg caused a reduction in fenoterol-stimulated heart rate that occurred immediately and remained low for the following 2 hours (n=3 rats). The binding of esmolol, a short acting ester-containingβ-blocker, (log KD-6.56 ± 0.03, n=34) was not affected by incubation with serum esterase (log shift = 0.01 ± 0.01, n=7) but co-incubation with liver esterase caused a 645-foldparallel rightward shift of the competition binding curve (log shift = 2.81 ± 0.06, n=19) consistent with significant esmolol hydrolysis. Incubation with 10% human serum did not cause any shift of the esmolol binding curve (0.04 ± 0.07, n=6). Following iv injection in rats (3mg/kg, a dose equipotent to that used for betaxolol), esmolol caused a similar reduction of fenoterol-stimulated tachycardia that wore off over time such that heart rate had returned to baseline by 40 minutes (n=4 rats). NDD-446 had a log KD value for the β1-adrenoceptor of-7.71 ± 0.03, n=35. Co-incubation with both liver esterase and serum esterase caused a rightward shift of the binding curve (log shift 2.79 ± 0.07, n=8 and 2.45 ± 0.10 n=4 respectively). Incubation with 10% human serum also caused a rightward log shift (2.60 ± 0.10, n=6). When injected into rats (0.3mg/kg, the equipotent dose to that used for betaxolol, n=5 rats, and at 3mg/kg, 10-times the equipotent dose, n=5 rats), NDD-446 did not cause any decrease in fenoterol-stimulated tachycardia. In summary, betaxolol, a non-ester containing β-blocker, was totally insensitive to co-incubation with esterases. In contrast, esmolol was found to be sensitive to liver esterase but not to serum esterase or human serum. Liver hydrolysis of esmolol is therefore a likely explanation for the short duration of its systemic β-blocking effect following iv injection in rats. NDD-446 however, was sensitive to both serum and liver esterase (and human serum) and as a consequence was inactivated immediately upon iv injection into rats and did not cause any β-blocking reduction in heart rate. Thus, by altering the esterase selectivity, the iv duration of esterase-sensitive drugs can be altered. Candidate β-blockers, such as NDD-446, that are sensitive to serum (and liver) esterases and are hydrolysed immediately upon entry into the blood stream are likely to result in topical agents truly devoid of systemic side-effects. Baker JG et al., (2011) FASEB J 25: 4486-4497. Léauté-Labrèze C et al., (2008) N Engl J Med 358: 2649-2651. Filippi L et al., (2013) J Pediat. 163: 1570-1577.
|