Print version
Search Pub Med
Effect of cryopreservation on an autologous PBMC: endothelial cell bioassay to detect cytokine storm causing therapeutic antibodies.
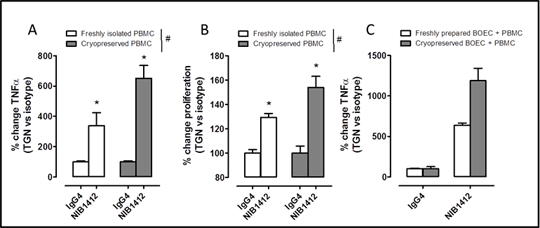
Biologics, including monoclonal antibodies, are a major focus of the pharmaceutical industry. Since biologics are highly specific, human tissue bioassays for the testing of efficacy and safety are increasingly important. Of particular concern is the potential for biologics to cause cytokine storm reactions, which in the case of TGN1412 for example, were not detected in animal models (1). We have recently reported a novel and superior cytokine-storm bio-assay that detects TGN1412(2) using endothelial cells grown from human blood (blood outgrowth endothelial cells; BOECs) cultured with PBMCs from the same donor, thereby removing any immunological artefacts associated with mixing cells from separate donors. However, this assaycurrently relies on lengthy cell culture steps and repeated visits of donors. In order to improve the utility of this assay we have investigated the effects of cryopreservation of PBMCs and BOECs on their responses to an antibody identical to TGN1412 (NIB 1412) (3). PBMCs and BOECs were isolated as described previously (2) and either used without (freshly prepared) or after cryopreservation. Cells were cryopreserved in liquid nitrogen before being thawed and resuspended as described previously (2) in RPMI for PBMCs and Lonza-EGM2 for BOECs. PBMCs were added to immobilised NIB1412 (1µg/well) or to autologous BOECs followed by the addition of NIB1412(1µg/well) in aqueous phase and incubated for 24h. TNFα release and proliferation were measured by ELISA and Alamarblue® assay respectively. As shown previously (4) freshly isolated PBMCs were stimulated to release TNFα and to proliferate when exposed to NIB1412 immobilised on plastic (Figure 1A and B). Cryopreserved PBMCs also responded to immobilised NIB1412. Importantly, cryopreserved autologous co-cultures of PBMCs and BOECs not only responded to NIB1412 but also displayed a greater percentage increase in TNFα than similarly treated cells isolated and/or cultured without cryopreservation. This data indicates that both PBMCs and BOECs from the same donor can be cryopreserved and used as an ‘off-the-shelf’ cytokine storm testing assay. This assay format will allow for future development and provides a more convenient platform for the testing biological drugs by the pharmaceutical industry.
Figure 1. Effect of cryopreservation and recovery from liquid nitrogen on TGN1412 sensing by PBMCs. Freshly prepared or cryopreserved PBMCs were added to either (A and B) immobilised NIB1412 (1μg/well; 250μl) or to (C) freshly isolated or cryopreserved same donor blood outgrowth endothelial cells (BOEC) followed by incubation with NIB1412 (1μg/well; 250μl). TNFα (A and C) and proliferation (B) was measured as readouts. Statistical significance for the effect of NIB1412 was determined by one-sample t-test vs. a theoretical value of 100, and between assays freshly isolated or cryopreserved cells was determined by two-way ANOVA (A and B; n=18 from 6 donors; #p<0.05), statistical testing was not carried out on data in panel C (n=3 from one donor). Data are mean ± SEM. Isotype/basal levels: A Fresh 1274±285.6 pg/ml, Cryopreserved 437±73.3 pg/ml; C Fresh 51±4.4 pg/ml, Cryopreserved 21±5.5 pg/ml.
References (1) Sutharalingham et al. (2006) NEJM 355: 1018-28; (2) Reed et al. (2015) FASEB J 29: 2595-2602; (3) Ball et al. (2012) J Immunol 189: 5831-5840; (4) Eastwood et al. (2013) Br J ClinPharmacol 76: 299-315
|