Potentiation of purinergic ion channels by ginsenosides; investigating selectivity and molecular binding site
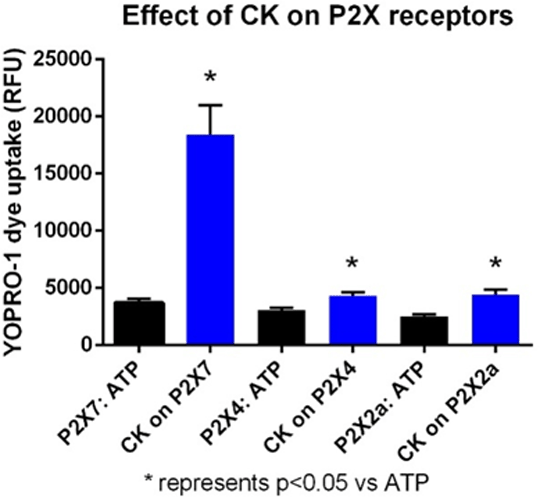
Ginseng is a medicinal herb used in Asian cultures for thousands of years as an “all healing” precious tonic thought to have many beneficial effects including stimulating the immune system. Many chemicals are present in ginseng including pharmacologically active ginsenosides. These glycosylated steroid-like chemicals have been reported to have multiple actions on cells including cytotoxicity and immuno modulatory effects. We recently demonstrated that several protopanaxdiol (PPD) ginsenosides potentiate ATP responses at the purinergic ion channel P2X7 (1). In this study we have investigated the selectivity of ginsenosides for P2X7 within the purinergic channel family to begin to establish a molecular binding site for these positive allosteric modulators. Stable cell lines expressing human P2X4 or human P2X2a were established in HEK-293 cells and utilised in a YOPRO uptake screening assay on a Flexstation 3 plate reader. Data is expressed as mean area under dye uptake curve+/- SEM and was analysed for significance using ANOVA with Sidak’ spost-test for multiple comparisons (Graph Pad Prism v5). In HEK-rat P2X7 cells we saw the same rank order of effect for PPD ginsenosidesas in HEK-hP2X7 cells (CK=Rd>Rb1>Rh2) with CK exhibiting a 5-fold potentiation of response to 200 µM ATP. We established that the ATP-induced YOPRO assay was robust for measuring P2X4 responses in HEK293 cells. In HEK-hP2X4 cells the known positive allosteric modulator ivermectin was shown to increase ATP responses (146%, P<0.05, n=12 wells) and the P2X4 antagonist 5-BDBD reduced ATP responses by 40% (P<0.05, n=8 wells). The EC50 for ATP-induced YOPRO uptake at human P2X4 was 2 µM (n=11 experiments). Ginsenosides showed a different rank order of potentiating effect at P2X4 (Rh2>CK>Rd>Rb1). CK potentiated the ATP response on average by 1.5-fold (n=4 experiments) whereas Rh2 potentiated by 2.5-fold (n=2 experiments). In HEK-hP2X2a cells we observed no inhibition by the P2X7 inhibitor AZ11645373 or the P2X4 inhibitor 5-BDBD. In these cells ginsenoside CK potentiated ATP responses, by mean of 1.6-fold (n=3 experiments). This data suggests that ginsenoside CK may not be selective for P2X7 with this modulator affecting both P2X4 and P2X2 responses to some extent. However, the largest effect is demonstrated at P2X7 receptors (Fig. 1). Using this selectivity information we can begin to investigate aginsenoside binding site on P2X7. Molecular modelling and docking experiments have predicted a binding site on P2X7 which will be further verified by site-directed mutagenesis.
(1) Helliwell et al, 2015, Br J. Pharm. 172: 3326-40.
|