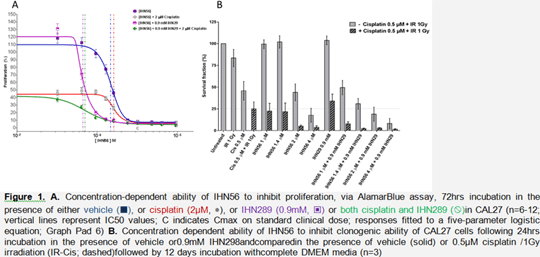
The Accelerated drug redeployment platform successfully employed to identify drug combination IHN56 and IHN29for Head and Neck Cancer treatment.
Head and neck cancer (HNC) is the 6th most common cancerwith incidence rate rapidly rising. Patient outlook is poor with late stage disease havinga high probability of relapse. The reason for poor outlook is that current treatments are ineffective withconsiderable side effects. Hence there is an urgent need for the discovery of new more targeted better tolerated HNC therapies. We have developed a drug discovery and redeployment platform coined “Accelerated”. Acceleratedcomprises a number of stages. Stage1, an initial high throughputscreening assays on a panel of representative HNC cell lines. Stage 2, validation by additionalin vitro assays on bothcell lines and primary cultures. In Stage 3, in vitro findings are validatedusingxenograft animal models. Stage 4 is forinitiating and running early to late phaseclinical trials. Using our Accelerated platform we screened a panel of 100 off-patent licensed drugs atclinicallyachievable concentrationsusing a panel of 3HNC cell lines (SCC040, Cal27 and VU147) identifying a number ofleads. The strongest hitwasa combination of drug IHN56 (3.2μM) and INH29 (0.6mM) exhibiting significant anti-proliferative (Figure 1A; 8% compare to control), anti-clonogenic (Figure 1B; 8.1% compared to control) and pro-apoptotic (data not shown; DNS) activity against CAL27 (p53 WT, HPV-) cells. Same was true in SCC040 (p53 mutant, HPV-) and VU147 (p53 mutant, HPV+) cells (DNS), with the combination being better than either drug alone on all our assays. Moreover, we demonstrated that this drug combination is comparable to standard treatment, cisplatin and irradiation (Figure 1A and 1B). Notably, both candidate drugs are used in clinical practice at equivalent concentrations with minimal toxicity and preliminary xenograft animal experiments using NSGmale micehave demonstrated the combination is well tolerated (DNS). In summary we have demonstrated in vitro using established and primary cell lines that our drug combination identified using the Acceleratedplatform is effective in inhibiting proliferation and clonogenic ability and inducing apoptosis in a dose dependent manner.
|