IgG and Serum Albumin Overcome Steric Constraints to Bind the Neonatal Fc Receptor in the Membrane
The Neonatal Fc Receptor (FcRn) binds IgG and Albumin in the endosome, diverting them away from the lysosomes and recycling them to the cell surface. The complex forms in a pH dependent manner. The low pH of the endosome protonates Histidine, causing salt bridges to form, while the neutral pH at the cell surface breaks these interactions. FcRn resides on an 8 residue linker separating the extracellular domain from its transmembrane helix. We hypothesised that the proximity of the membrane may restrict concurrent binding of both IgG and albumin, hindering or completely preventing formation of the ternary complex. To test this we studied binding to FcRn by flow cytometry. For both proteins pH dependent binding was observed confirming FcRn as the binding partner. Under equilibrium conditions a Kd for labelled IgG1 was measured in the presence of varying concentrations of albumin whilst a labelled albumin tracer was used to confirm albumin binding. Binding of both proteins was observed, indicating that the ternary complex can form within the membrane.
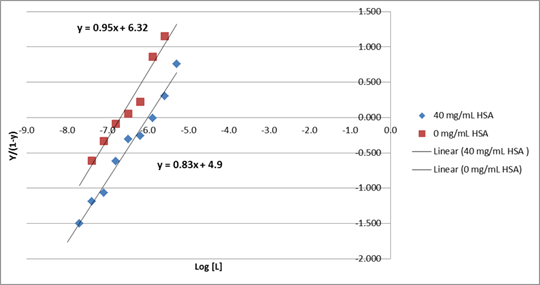
Figure 1. Hill Plot of IgG1 binding to FcRn in the presence and absence of albumin.
In the absence of albumin IgG1 Kd was 137nM. At 40mg/mL of albumin, the middle of the physiological range, the Kd fell to 892nM. A hill plot of these data suggests that the binding of Albumin and IgG may not constitute mutually independent events and that, within the membrane environment, the two events may be negatively cooperative, indicated by the hill slope reducing in the presence of albumin. By combining public crystal structures we modelled the ternary complex seated within a course-grain membrane using molecular dynamics and the Martini force field. To accommodate binding of both proteins the model showed significant re-arrangement which may explain our in vitro observations.
|