Print version
Search Pub Med
In vitro modelling of Loin Pain Haemauturia Syndrome (LPHS)
Loin Pain Haematuria Syndrome (LPHS) may be considered one of the most painful human conditions. Primary LPHS comprises severe flank pain accompanied by haematuria or erythrocyte urine cytology without a primary cause such as renal casts, glomerular nephritis, or infection. Haematuria is reduced by ACE-inhibitors, but pain can often be refractory to opiates leading to desperate measures such as renal denervation or autotransplantation. We recently reported successful treatment of LPHS with the PDE5 inhibitor tadalafil (Russell et al., 2015). We wished to model the actions of blood on ureteral function with an aim of determining outcomes for pharmacological investigation, including tadalafil. Paired ureters from 25-350g male rats were mounted at 0.2g in 95%CO2/5%O2 gassed Krebs Hensleit buffer at 37oC. One was infused with blood via the renal calyx. Ureters were electrically stimulated using chick biventer electrodes placed proximally using a Grass S88 square wave stimulator. Pulses of 0.2ms width of 10 seconds at 20 or 40Hz, at 5-70V were given. Ureters were contracted at start and end of the experiment with 80mM KCl to determine maximal contraction and viability. Force of contraction (FoC, g), duration of relaxation post contraction (s), and area under the curve (AUC, g.s) were measured using AD Instruments Lab chart v.8, and paired ureters with and without blood compared. Conditions for determining renal calyx pacemaker activity were explored. Significance was assessed by 2-way ANOVA (Graph pad Prism v6).
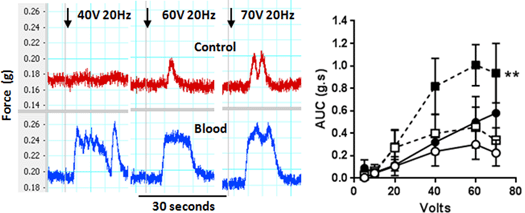
Figure 1. Left: Example trace showing force of contraction vs. Time (s) of paired electrically stimulated rat ureters, top: control, bottom: blood infused. Increasing voltage at 20 or 40Hz. Right Volt response (AUC, g.s) curve of paired rat ureters with 20Hz (circles), 40Hz (squares) without (open) and with (filled) blood. Ureters contracted to electrical stimulation at 20Hz and 40 Hz in a voltage response fashion reaching a maximum force of 0.02±0.008g at 60V with 20Hz frequency, and with greater force at lower voltage at 0.029±0.016 at 20V at 40Hz (p<0.05). The inclusion of blood increased (NS) maximum FoC to 0.035±0.14g (20Hz) and 0.054±0.015g. Control maximum duration at 60V 20Hz increased from 12.3±6.4s to 27.1±10.9s (NS, with blood) and unchanged at 60V 40Hz at 45±8.1s (without blood) and 39.3±10.5 (with blood). Control AUC at 20Hz was 0.30±0.13 increased to 0.50±0.30g.s. (NS) and at 40Hz from 0.45±0.18 to 1.01±0.18 (p<0.01, Fig. 1). Renal calyx pacemaker activity was observed if the kidney was retained with the ureters, and the bath washed frequently. Blood has been considered a bystander in LPHS, we have shown it increases contractile behaviour which could contribute to ureteral pain. We have developed an in vitro model with which the pharmacology of LPHS may be investigated. Russell, A., Chatterjee, S., Seed, M., et al. (2015) BMJ Case Reports doi: 10.1136/bcr-2014-209165
|