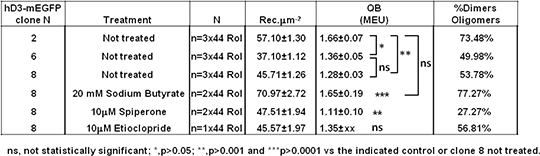
Defining the quaternary organization of the human dopamine D3 receptor and its regulation by receptor antagonist ligands The dopamine D3 receptor is a class A rhodopsin-like G protein-coupled receptor (GPCR) primarily coupled to inhibitory G-proteins. It has been reported that the human D3 receptor (hD3R) can form dimers and oligomers and we have previously defined the residues from within each of transmembrane domains (TMs) I, II, IV, V, VI and VII, as well as the intracellular helix VIII involved in the formation of the interfaces that allow hD3R monomers to interact and form two distinct dimeric and a ‘rhombic’ tetramer species (1).The relative abundance of different hD3R species at the cell surface of mammalian cells was here investigated to define the steady-state proportion of each form and to determine whether this can be regulated by cellular challenges. To do so we adopted a method that combines confocal microscopy imaging and spatial intensity distribution analysis (SpIDA) (2). This method is based on fitting fluorescence intensity histograms obtained from regions of interest (RoI, selected within single images) to obtain density maps of fluorescent molecules along with their quantal brightness (QB), which indicates their oligomeric state. We generated Flp-InTM T-RExTM 293 cells stably and constitutively expressing hD3R modified at the C-terminus by the incorporation of monomeric EGFP (hD3-mEGFP) and selected three different clones exhibiting different hD3R expression levels (Table 1). SpIDA on these clones showed a QB (expressed as monomeric equivalent units, MEU (3)) indicating that hD3R-mEGFP was present as a mixture of monomers and dimer/oligomers at the cell surface and that the size of the complexes and the percentage of dimer/oligomers increased with receptor density (Table 1).To test the hypothesis of a dependency between the proportion of dimer/oligomers and the receptor density we treated clone 8 cells with 20 mM sodium butyrate with the aim of enhancing hD3-mEGFP receptor expression level. SpIDA revealed that the number of receptor per μm-2 increased 1.5 times after sodium butyrate treatment and concurrently so did the complexity of the quaternary structure of hD3R and the percentage of dimer/oligomers at the cell surface (Table 1). Subsequently we tested whether dopamine receptor ligands could influence the quaternary structure of hD3R. SpIDA showed that long-term treatment with the selective hD3R antagonists etioclopride (10μM) or spiperone (10μM) did not significantly alter expression levels of hD3R but although etioclopride treatment did not alter the quaternary structure of hD3R spiperone treatment did, favouring the monomeric state (Table1). Table 1 Summary of SpIDA results
Our data provides further evidence of the co-existence of different hD3R species at the cell surface of mammalian cells. The equilibrium among these distinct forms can vary with the receptor density and can be modulated by receptor ligand binding. Ongoing studies are aimed to selectively disrupt the quaternary structure of the hD3R to determine the functional role of the different species in the signalling of this GPCR. (1) Marsango S et al. (2015). J Biol Chem 290: 14785–14796. (2) Godin AG et al. (2011), Proc. Natl. Acad. Sci. U S A 108: 7010–7015. (3) Pediani JD et al. (2016) J Biol Chem (in press).
|