The effect of the N-alkyl moiety on the interaction of pindolol analogues with the two agonist conformations of the human β1-adrenoceptor There are two active conformations of the β1-adrenoceptor (AR): a high affinity conformation (HAC) where responses are readily inhibited by antagonists and a low affinity conformation where agonist responses are relatively resistant to antagonism (1). Some ligands, e.g. pindolol, stimulate both conformations (2). Analogues of pindolol were synthesised to examine the contribution of the N-alkyl moiety to activation at each conformation of the human β1-AR. 3H-CGP 12177 whole cell binding and CRE-SPAP reporter gene assay were examined in cells stably expressing the human β1-AR as previously described (2).
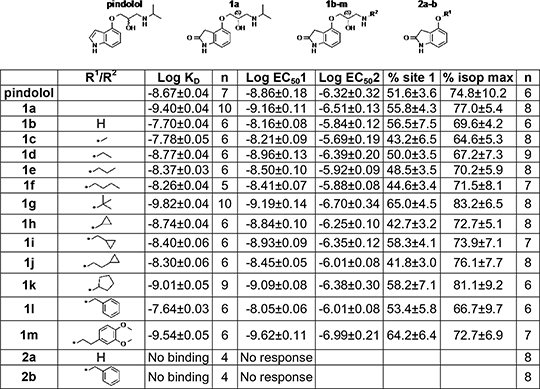
Table. Log KD values from 3H-CGP12177 binding and log EC50 values and % maximum isoprenaline responses from CRE-SPAP production. Values are mean±sem of n separate experiments
Ligand affinity for the HAC was affected by the nature of the N-alkyl moiety. Whilst an amine moiety was essential for receptor interaction, sterically small amine groups (e.g. 1b, 1c) gave lower affinity. Steric bulk near to the amine or a distant aromatic group, maintained high affinity for the HAC in addition to agonist activity at both conformations (1a, 1g, 1h, 1k, 1m). The nature of the N-alkyl moiety does not appear of importance in agonist stimulation at either conformation of the β1-AR. (1) Kaumann and Molenaar (2008). Pharmacol. Ther. 118: 303–336. (2) Baker et al. (2003). Mol. Pharmacol. 63: 1312–1321.
|