The effect of alterations to the N-alkyl moiety and benzothiazolone on affinity, efficacy and selectivity of the β2-agonist S1319 at the human β1- and β2-adrenoceptors S1319 was originally isolated from the marine sponge Dysidea sp. and has several structural similarities to adrenaline and salbutamol. It has been reported to have affinities of 120nM and 50nM for the β1 and β2-adrenoceptor(AR)s respectively but very high potency (log EC50 -10.6) for β2-mediated tracheal relaxation compared to salbutamol (Suzuki, et al., 1999a and b). The aim of this study was to investigate whether alterations to the N-alkyl group and the addition of an isopropyl group on the benzothiazolone affected the affinity, efficacy or selectivity of S1319 derivatives for the human β1 and β2-ARs. In this study, the parent compound S1319 and several analogues were synthesized. These were investigated using 3H-CGP12177 whole cell binding and CRE-SPAP gene transcription in CHO cells stably expressing a CRE-SPAP reporter gene and either the human β1 or β2-AR (Baker, 2010a and b).
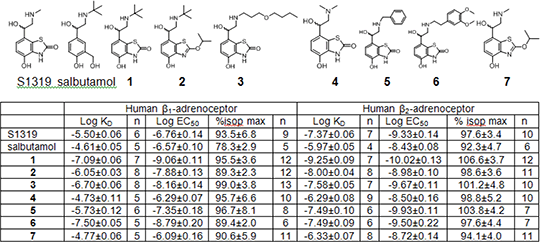
Table. Log KD values from 3H-CGP12177 binding and log EC50 values and % maximum isoprenaline responses from CRE-SPAP production. Values are mean±sem of n separate experiments.
S1319 was confirmed to be a β2-selective ligand with a 74-fold increase in affinity for the β2-AR over the β1-AR. It was also confirmed to be a full agonist at both receptors, with high potency for the β2-AR. The addition of the tert-butyl group (1) increased the affinity of the molecule for both receptors by 30- to 100- fold, however the addition of an ether side-chain (3) or benzyl substituent (5) had little effect on the affinity or efficacy. Alteration of the benzothiazolone head group to the isopropoxy analogue (2, 7) decreased the affinity and potency at both receptors relative to their parent compounds (1, S1319). All of the synthesised ligands retained full agonism compared with isoprenaline. Baker JG (2010a) Br. J. Pharmacol 160: 148–161. Baker JG (2010b) PLoS ONE 5(11): e15487. Suzuki et al., (1999a) Bioorganic and Med Chem Lett 9: 1361–1364. Suzuki et al., (1999b) Br. J. Pharmacol 128: 716–720.
|