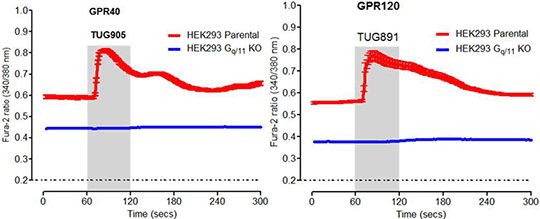
Desensitisation of the long chain fatty acid receptors FFA1 and FFA4 The G protein-coupled receptors FFA1 (GPR40) and FFA4 (GPR120) are very attractive targets for drug development for the treatment of both various metabolic disorders, specifically type-2 diabetes, and inflammatory conditions (1). Both receptors are coupled to Gq/11 and β-arrestin mediated pathways, which interact to influence systemic metabolic function in physiological and pathophysiological conditions (2). Following agonist binding, these receptors are phosphorylated by members of the G protein-coupled receptor kinases (GRK) family and also potentially by protein kinase C. Such effects frequently allow for recruitment of β-arrestins, receptor internalisation, and desensitisation. Agonist activation of these receptors can result in rapid desensitisation (3); however, mechanisms responsible are yet to be fully elucidated. Herein, human FFA1 and FFA4 receptors were expressed in both HEK293 cells and in modified HEK293 cell lines, generated by CRISPR-Cas9-mediated genome editing (4), that lacked expression of either Gq/11 or β-arrestin1/2 proteins as determined by immunoblot analysis. In cells lacking Gq/11 selective agonists of either FFA1 (TUG-905) or FFA4 (TUG891) receptors failed to produce responses in cell population or single cell calcium imaging studies. This was also the case in myo-inositol-1-phosphate (IP1) accumulation assays. However, such signals were regained when Gq alpha protein was re-introduced into the Gq/11 knockout cell lines. Experiments in β-arrestin1/2 knockout cells demonstrated that lack of these arrestins did not prevent rapid agonist-mediated desensitisation of either FFA1 or FFA4 and that both receptors were as capable of elevating [Ca2+]i in these cells as in the parental cell lines. These results suggest that non β-arrestin- mediated pathways are likely involved in the desensitisation process.
Although both FFA1 and FFA4 are able to engage β-arrestins in an agonist-dependent manner, maintained rapid desensitisation in the absence of β-arrestins indicates potential roles of a second messenger-dependent kinase, e.g. protein kinase C in the regulation of signalling and function of these receptors (5) and this could help better understanding the desensitisation of these important therapeutic targets. (1) Milligan G et al. (2015). Br J Pharmacol. 13:3254–3265; (2) Moran BM et al. (2016) Acta Diabetol. [Epub ahead of print] PMID: 26739335; (3) Hudson BD et al. (2013). Mol Pharmacol. 5:710–725; (4) Schrage R et al. (2015). Nat Commun. 6:10156; (5) Sosa-Alvarado C et al. (2015) Eur J. Pharmacol. 768, 108–115.
|