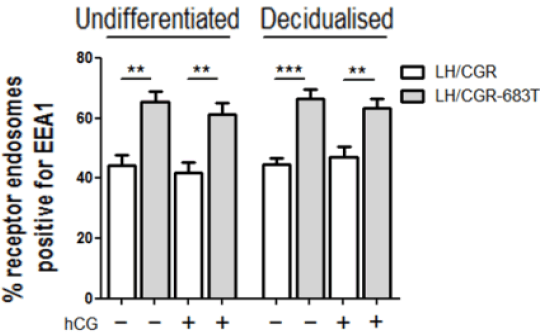
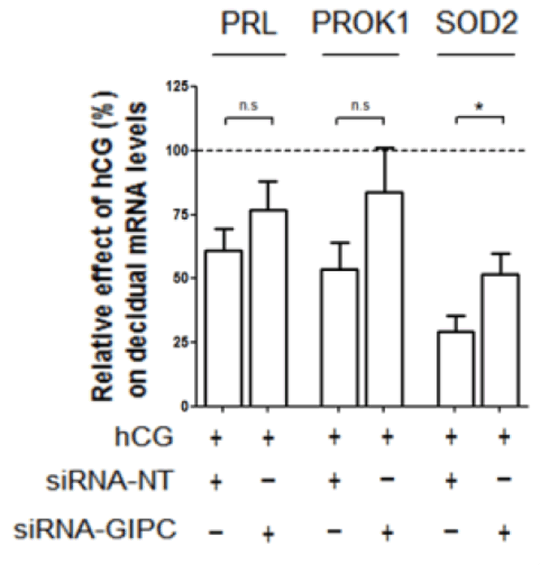
The Role of the Very Early Endosome in the trafficking of the LH/CGR and hCG-mediated signalling in human endometrial stromal cells (HESCs) The glycoprotein hormone human chorionic gonadotrophin (hCG) is known to be essential during early pregnancy to maintain progesterone production from the corpus luteum. It also acts in a paracrine manner signalling directly to the uterine endometrium to modulate inflammatory, apoptotic, differentiation and oxidative stress responses. hCG is known to signal via its GPCR the luteinising hormone/chorionic gonadotrophin receptor (LH/CGR), which is known to be expressed in the endometrium. In HEK293 cells, the LH/CGR is known to internalise to the Very Early Endosome (VEE), an endosomal sub-compartment distinct from the classical early endosome (EE), and this compartment is marked by the adaptor protein APPL1 but devoid of the EE marker EEA1. Mechanistically, the localisation of the LH/CGR to the VEE is dependent on the interaction with the PDZ protein GIPC (GAIP-interacting protein C-terminus) and is essential for its recycling and MAPK signalling (1). Here, we explore the relevance of the VEE, and in particular the role of GIPC, in LH/CGR trafficking in primary human endometrial stromal cells (HESCs) and how this compartment may govern hCG signalling. In order to assess the trafficking and subcellular localisation of the receptor, HESCs isolated from endometrial biopsies taken from control patients (n=3) were transfected with the full-length LH/CGR or a mutant form of the receptor lacking the last 17 residues of the C-terminal tail that is unable to bind GIPC (LH/CGR-683T). HESCs were subsequently differentiated (decidualised) with cAMP (0.5mM) and progesterone (1μM) analogues or left undifferentiated. Co-localisation for the EE marker EEA1 with each receptor was then assessed prior to and after hCG addition. The LH/CGR-683T displayed a significantly higher amount of co-localisation with EEA1 than the full-length receptor (Fig. 1), suggesting that this region of the C-terminal tail is required to route the receptor away from the EE. We also observed co-localisation of GFP-tagged GIPC in receptor endosomes (data not shown). Preliminary experiments also investigated the effect of GIPC knockdown on cell differentiation by measuring levels of the differentiation markers prolactin (PRL), prokineticin 1 (PROK1) and superoxidase dismutase 2 (SOD2) (n=3). Results indicate that absence of GIPC reverses the hCG-induced downregulation of SOD2, where in control cells hCG caused a 70.7 ± 6.0% decrease and in GIPC depleted cells that reversal was reduced to 48.2% ± 7.8% (Fig. 2).
Figure 1. LH/CGR-683T localises to a larger proportion of EEA1 positive endosomes
Figure 2. Effect of GIPC knockdown on expression of decidual gene markers In conclusion, this data indicates that GIPC could be playing a crucial role in the trafficking of the LH/CGR and, in addition, endogenous hCG-induced downstream signalling to regulate endometrial decidualisation and the implantation window. (1) Jean Alphonse et al. (2014) J Biol Chem, 2014. 289(7): p. 3960–77.
|