Print version
Search Pub Med
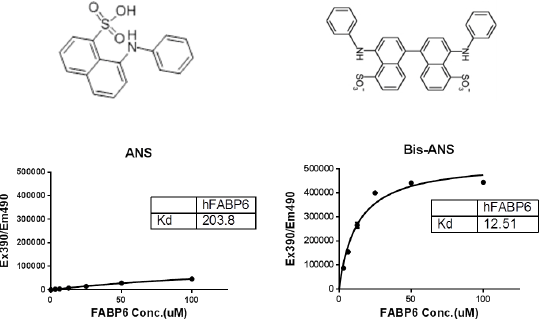
Comparison of orthogonal assays in the study of the bile-salt binding properties of FABP6 FABP6 is one of nine fatty acid binding proteins (FABPs) that constitute a family of low molecular weight proteins that are capable of binding hydrophobic ligands. Each FABP has a unique expression pattern that reflects the high level of lipid metabolism of the tissues in which it is found. While most FABPs function to promote intracellular fatty acid solubilization, trafficking and metabolism, one particular member, FABP6 (also known as the ileal bile acid binding protein IBABP), shows a strong preference for binding bile salts. Indeed, it is discretely and almost exclusively localised to enterocytes lining the ileum, where it is expressed at very high levels and is ideally located to play a role in the maintenance of bile acid homeostasis in the enterohepatic cycle. Interestingly, a mutation in FABP6 - Thr79Met - has been found to correlate with a reduced risk of type-II diabetes mellitus (T2DM) in obese individuals1, and in recent years a growing body of evidence has shown that bile acid metabolism is altered in both T2DM patients and animal models2,3. This makes FABP6 a potentially very interesting target for anti-diabetes drug discovery. The small, soluble nature of the protein lends itself to structure-based drug design in the forefront of the discovery process. A parallel approach was taken, using a novel Bis-ANS displacement assay along with surface plasmon resonance (SPR), to get a full understanding of the bile-salt binding properties of FABP6. This should give an excellent starting point for a fragment screen that will complement our structural studies and allow for rational structure-based drug design in the search for molecules that interact with this interesting target. Figure 1a: Bis-ANS displays saturation binding kinetics on human FABP6 with an apparent kd of 12.5µM Figure 1b: A range of bile salts are capable of displacing bound Bis-ANS from human FABP6
1) Fisher E1, Grallert H, Klapper M, Pfäfflin A, Schrezenmeir J, Illig T, Boeing H, Döring F. Evidence for the Thr79Met polymorphism of the ileal fatty acid binding protein (FABP6) to be associated with type 2 diabetes in obese individuals. Mol Genet Metab. 2009 Dec;98(4):400–5. 2) E. Andersen, G. Karlaganis, and J. Sjovall, “Altered bile acid profiles in duodenal bile and urine in diabetic subjects,” European Journal of Clinical Investigation, vol. 18, no. 2, pp. 166–172, 1988. 3) K. Suhre, C. Meisinger, A. Döring et al., “Metabolic footprint of diabetes: a multiplatform metabolomics study in an epidemiological setting,” PLoS ONE, vol. 5, no. 11, Article ID e13953, 2010.
|


.gif)