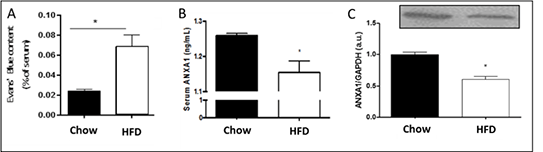
Blood-Brain Barrier Induced Leakage in Type 2 Diabetes: Molecular and Cellular Mechanisms Although recent studies have highlighted that DM is a risk factor to neurodegenerative disorders, the impact of type 2 DM (T2DM) on blood brain barrier (BBB), the interface between brain parenchyma and systemic circulation, remains poorly understood. The present study was designed in order to evaluate the potential beneficial effects of endogenous anti-inflammatory molecule Annexin A1 (ANXA1)1, an important component of BBB tightness, to preventing/repairing the T2DM-induced BBB dysfunction. C57/bl6 mice were fed with high fat diet (HFD group), a model of experimental T2DM, for 10 weeks. BBB permeability was determined by Evans Blue (EB) dye extravasation to brain parenchyma. Brains were collected and immunohistochemistry was performed to determine occludin and laminin expression, important components of BBB. Furthermore, brain capillaries and serum were isolated to determine ANXA1 levels by western blotting and ELISA, respectively. bEnd5, a murine brain endothelial cell line, were used to perform in vitro studies. Wild type (WT) and cells infected with short hairpin RNA (shRNA) to knockdown ANXA1 expression were incubated (16 hours) in normoglycaemic (NG - 5.5 mM) or hyperglycaemic (HG - 40 mM) conditions in presence or absence of recombinant ANXA1 (20 ng/ml). Protein expression of ANXA1 and glucose transporter GLUT1 on bEnd5 cells were determined by western blotting and endothelial metabolism (glycolysis and oxidative phosphorylation) was determined by Seahorse® assay. Data represents mean ± SEM. Analysis was performed using ANOVA or Student’s T test, as applicable, *P<0.05. HFD-induced T2DM increased BBB permeability (Figure 1A) and down-regulated occludin (Chow Diet (CD-Control) 100±4.8 vs HFD 82.8±4.2, n=3) and laminin (CD 100±4.8 vs HFD 71.1±4.7, n=3) expression in comparison to CD group. Furthermore, ANXA1 levels were down-regulated on both serum (Figure 1B, n=5) and brain capillaries (Figure 1C, n=5) from CD and HFD mice. bEnd5 WT exposed to HG showed down-regulated ANXA1 expression (NG 5.9±0.9 vs HG 3.4±0.3, n=3) and up-regulated GLUT1 expression in comparison to NG conditions (NG 0.15±0.04 vs HG 0.47±0.09, n=3).At NG conditions, WT and shRNA ANXA1 showed similar glycolysis and oxidative phosphorylation and treatment with ANXA1 reduced maximal glycolytic activity on both WT (WT 126.7±2.9 vs WT + ANXA1 94.9±8.4, n=3) and shRNA ANXA1(shRNA 110.1±7.8 vs shRNA + ANXA1 80.9±2.8, n=3) but had no effect on oxidative phosphorylation. At HG conditions, shRNA presented lower maximal glycolytic capacity (WT 82.5±0.4 vs shRNA 62.9±3.5, n=3) and higher maximal oxidative phosphorylation (WT 224±2.6 vs shRNA 273±6.6, n=3) but ANXA1 treatment had no effect on both parameters.
In conclusion, our data suggest that metabolic imbalance associated to T2DM down-regulates ANXA1 expression on BBB and ANXA1 is involved in regulation of brain endothelial cell metabolism. 1. Cristante et al. Identification of an essential endogenous regulator of blood brain barrier integrity: pathological and therapeutic implications. Proc Natl Acad Sci U S A. 110(3):832-41
|