| 007P London, UK Pharmacology 2016 |
Kainate Receptors: A potential therapeutic target for neuropsychiatric disorders
Introduction: Kainate receptors (KARs) are ionotropic glutamate receptors involved in brain function. KARs form functional ion channels from tetrameric combinations of five subunits (GluK1-5) and they are modulated by auxiliary proteins Neto1 and Neto2. In this project, we hypothesize that functional genetic variants within KAR and Neto genes contribute to risk or protection for developing neuropsychiatric disorders [1]. This study will investigate how such genetic risk factors and pharmacological compounds (antidepressants, antipsychotics) may affect KAR function.
Methods: We have used two complementary approaches to investigate the role of KARs in brain disease. The first approach consists of bioinformatic analysis of next generation sequencing data and interpreting how rare coding variants may contribute to neuropsychiatric diseases in patient populations. The second approach involves electrophysiological recording of Xenopus oocytes expressing cloned rat and human KARs and treatments with pharmacological agents such as ketamine and citalopram.
Results: We report the identification of a number of rare predicted damaging missense mutations which may affect the expression and the physiological properties of KARs. We also report our functional findings of GluK2 and GluK2/GluK4 subunits regarding their sensitivity to glutamate and kainic acid (KA) and how ketamine and citalopram affect KAR ionic currents. Citalopram is reported to have a stronger antagonistic effect on GluK2 compared to GluK2/GluK4 receptors, while ketamine seemed to be a weak antagonist on both types of KARs. We also show that Neto1 affects KA sensitivity of GluK2 (figure 1) and GluK2/GluK4 receptors whilst also being responsible for slower decay kinetics of both types of KARs.
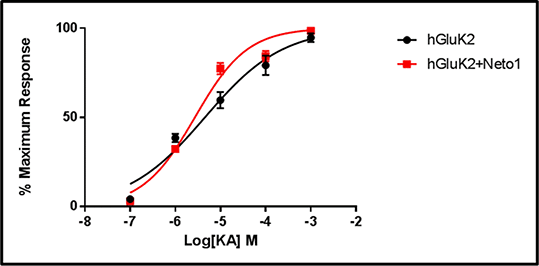
Figure 1: Concentration-response relationship of human homomeric GluK2 receptors with or without co-expression of human Neto1. Percentage of maximum response data were plotted and fitted by the Hill equation to estimate the EC50 for KA. EC50 values were 4.54 μM for human homomeric GluK2 receptors (95%Cl: 2.92 μM to 7.07 μM; n=5) and 2.72 μM human homomeric GluK2 receptors with Neto1 (95%Cl: 2.14 μM to 3.46 μM; n = 7-8)
Conclusion: Our current findings show that genetic variation within KARs and Neto genes is linked with neuropsychiatric phenotypes and that active compounds (e.g. antidepressants) can alter the pharmacological and electrophysiological properties of KARs. Future work will involve investigating differences in these properties between wild type and mutated KARs. This research will contribute to a better understanding of the link between genetic risk, biological processes and potential therapeutic avenues for brain diseases.
References
1. FRANK, R. A., et al., (2011). Clustered coding variants in the glutamate receptor complexes of individuals with schizophrenia and bipolar disorder. PLoS One 6(4): e19011.
2. KNIGHT, H. M. et al., GRIK4/KA1 protein expression in human brain and correlation with bipolar disorder risk variant status. Am J Med Genet B Neuropsychiatr Genet, 159: 1: 21-9, 2012. ISSN 1552-485X.

